

碳正离子Lewis酸催化氧化还原中性胺基α-C(sp3)H芳基化反应
收稿日期: 2015-09-06
网络出版日期: 2015-10-29
基金资助
项目受国家自然科学基金(Nos. 21390400, 21202170, and 21472193)和973项目(No. 2012CB821600).
Carbocation Lewis Acid Catalyzed Redox-Neutral α-C(sp3)H Arylation of Amines
Received date: 2015-09-06
Online published: 2015-10-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21390400, 21202170, 21472193) and 973 program (No. 2012CB821600).
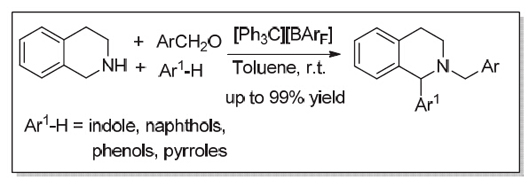
研究了碳正离子作为Lewis酸催化三组分氧化还原中性胺基α-C(sp3)-H芳基化反应. 在温和条件下, 三苯甲基溴和NaBArF原位生成的[Ph3C][BArF]可以高效、高化学选择性的催化四氢异喹啉、芳香醛和吲哚的氧化还原中性胺基α-C(sp3)-H芳基化反应(产率最高达99%). 并且其它的亲核试剂(如: b-萘酚、3-甲氧基苯酚、3-N,N-二甲基胺基苯酚和2,5-二甲基吡咯等)也能较好的完成该反应.
关键词: 碳正离子; Lewis酸; 氧化还原中性反应; α-C(sp3)H芳基化; 有机催化
张启超, 吕健, 罗三中 . 碳正离子Lewis酸催化氧化还原中性胺基α-C(sp3)H芳基化反应[J]. 化学学报, 2016 , 74(1) : 61 -66 . DOI: 10.6023/A15090587
The α-substituted nitrogen-containing heterocycles (N-heterocycles) like tetrahydroisoquinolines (THIQs) and piperidines are common in nature and are present as the key functional group in bioactive and pharmacologically active compounds. The two traditional approaches for the synthesis of α-substituted N-heterocycles are Pictet-Spengler condensation and hydrogenation of the α-substituted cyclic imines. Recently, direct C(sp3)-H functionalization of N-heterocycles with various nucleophiles catalyzed by transition metal complex under the oxidants presents an atom-economic protocol without prior installation of activating group. However, new catalytic strategy or catalysts are still desirable in order to further address the use of excess oxidants and expensive transition metal complex. Stable carbocations, such as tritylium ions, have been widely used as organic Lewis acid catalysts and reagents in organic synthesis. In our studies, it was found that tritylium salts [Ph3C][BArF] (10 mol%) in situ generated by Ph3CBr (0.02 mmol) and NaBArF (0.02 mmol) could promote the three component redox-neutral α-arylation of amines reaction with tetrahydroisoquinoline 2 (0.3 mmol), aldehyde 3 (0.2 mmol) and indole 4 (0.3 mmol) in toluene (1.0 mL) to afford the desired α-substituted N-heterocyclic compounds 5 in good yields (up to 99% yield) at ambient temperature to 50 ℃. To our satisfaction, a variety of differently substituted indoles 4a~4i could work well with tetrahydroisoquinoline 2 and 2,6-dichlorobenzaldehyde 3a to provide the corresponding α-substituted N-heterocyclic compounds 5a~5i in good to excellent yields at room temperature. When other substituted benzaldehydes 3b~3f were used, the reactions could also proceed smoothly to give the compounds 5j~5o with moderate yield (up to 66% yield) at 50 ℃. Moreover, we have also developed redox-neutral α-arylation of tetrahydroisoquinoline 2 with other aromatic nucleophile such as substituted phenol, b-naphthol and pyrrole under the optimal reaction conditions. Importantly, the model reaction, conducted in the presence of Na2CO3(1.0 equiv.) or 2,6-bis(tert-butyl) pyridine (0.5 equiv.) at room temperature in toluene also proceeded smoothly to give the desired adduct 5a with comparable yields (82% and 92% yield, respectively), which could exclude the possibility of free acid catalysis in this reaction.

[1] (a) Shamma, M.; Moniot, J. L. The Isoquinoline Alkaloids, Chemistry and Pharmacology, Academic Press, New York and London, 1972;
(b) Hagel, J. M.; Facchini, P. J. Plant Cell Physiol. 2013, 54, 647.
[2] (a) Stöckigt, J.; Antonchick, A. P.; Wu, F.; Waldmann, H. Angew. Chem. Int. Ed. 2011, 50, 8538;
(b) Rozwadowska, M. D. Heterocycles 1994, 39, 903;
(c) Czarnocki, Z.; Siwicka, A.; Szawkalo, J. Curr. Org. Synth. 2005, 2, 301.
[3] For selected reviews of direct sp3 C—H bond activation adjacent to nitrogen atoms: (a) Campos, K. R. Chem. Soc. Rev. 2007, 36, 1069;
(b) Mitchell, E. A.; Peschiulli, A.; Lefevre, N.; Meerpoel, L.; Maes, B. U. W. Chem. Eur. J. 2012, 18, 10092;
(c) Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242;
(d) Peng, H. M.; Dai, L.-X.; You, S.-L. Angew. Chem. Int. Ed. 2010, 49, 5826;
(e) Yang, L.; Huang, H. Catal. Sci. Technol. 2012, 2, 1099;
(f) Pan, S. C. Beilstein J. Org. Chem. 2012, 8, 1374;
(g) Qin, Y.; Lv, J.; Luo, S. Tetrhedron Lett. 2014, 55, 551;
(h) Zhang, Y.; Feng, B. Chin. J. Org. Chem. 2014, 34, 2406. (张艳, 冯柏年, 有机化学, 2014, 34, 2406.);
(i) Tan, M.; Gu, Y.; Luo, X.; Zhang, P. Chin. J. Org. Chem. 2015, 35, 781. (谭明雄, 顾运琼, 罗旭健, 张培, 有机化学, 2015, 35, 781.) For selected examples:
(j) Zhang, Y.; Luo, S.; Feng, B. Chin. J. Org. Chem. 2014, 34, 2249 (张艳, 罗莎, 冯柏年, 有机化学, 2014, 34, 2249.);
(k) Xia, X.; Li, L.; Liang, Y. Chin. J. Org. Chem. 2013, 33, 675. (夏晓峰, 李莲花, 梁永民, 有机化学, 2013, 33, 675.)
[4] For reviews of CDC reactions, see: (a) Li, C.-J. Acc. Chem. Res. 2009, 42, 335;
(b) Yoo, W.-J.; Li, C.-J. Top. Curr. Chem. 2010, 292, 281;
(c) Scheuermann, C. J. Chem. Asian J. 2010, 5, 436;
(d) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215;
(e) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780;
(f) Shi, W.; Liu, C.; Lei, A. Chem. Soc. Rev. 2011, 40, 2761;
(g) Wenlandt, A. E.; Suess, A. M.; Stahl, S. S. Angew. Chem., Int. Ed. 2011, 50, 11062;
(h) Klussmann, M.; Sureshkumar, D. Synthesis 2011, 353;
(h) Li, Y.; Ma, L.; Li, Z. Chin. J. Org. Chem. 2013, 33, 704 (李远明, 马丽娜, 李志平, 有机化学, 2013, 33, 704.)
[5] For reviews of redox-neutral approach to C-H Functionalization, (a) Seidel, D. Acc. Chem. Res. 2015, 48, 317;
(b) Peng, B.; Maulide, N. Chem. Eur. J. 2013, 19, 13274.
[6] (a) Naredla, R. R.; Klumpp, D. A. Chem. Rev. 2013, 113, 6905;
(b) Stang, P. J. In Carbocation Chemistry, Eds.: Olah, G. A.; Prakash, G. K. S., Wiley, Hoboken, NJ, 2004, pp. 1~6.
[7] (a) Norris, J. F. Am. Chem. J. 1901, 25, 117;
(b) Kehrman, F.; Wentzel, F. Chem. Ber. 1901, 34, 3815.
[8] For some recent examples, see: (a) Ludwig, M.; Hoesl, C. E.; Höfner, G.; Wanner, K. T. Eur. J. Med. Chem. 2006, 41, 1003;
(b) Sokol, J. G.; Cochrane, N. A.; Becker, J. J.; Gagné, M. R. Chem. Commun. 2013, 49, 5046;
(c) Wan, M.; Meng, Z.; Lou, H.; Liu, L. Angew. Chem. Int. Ed. 2014, 53, 13845;
(d) Chen, W.; Xie, Z.; Zheng, H.; Lou, H.; Liu, L. Org. Lett. 2014, 16, 5988;
(f) Song, G.; Wylie, W. N. O.; Hou, Z. J. Am. Chem. Soc. 2014, 136, 12209.
[9] (a) Mukaiyama, T.; Kobayashi, S.; Murakami, M. Chem. Lett. 1984, 13, 1759;
(b) Mukaiyama, T.; Kobayashi, S.; Murakami, M. Chem. Lett. 1985, 14, 447;
(c) Kobayashi, S.; Matsui, S.; Mukaiyama, T. Chem. Lett. 1988, 17, 1491;
(d) Mukaiyama, T.; Kobayashi, S.; Shoda, S.-L. Chem. Lett. 1984, 13, 907;
(e) Kobayashi, S.; Murakami, M.; Mukaiyama, T. Chem. Lett. 1985, 14, 953;
(f) Bah, J.; Franzén, J. Chem. Eur. J. 2014, 20, 1066;
(g) Bah, J.; Naidu, V. R.; Teske, J.; Franzén, J. Adv. Synth. Catal. 2015, 357, 148.
[10] Xie, Z.; Liu, L.; Chen, W.; Zheng, H.; Xu, Q.; Yuan, H.; Lou, H. Angew. Chem. Int. Ed. 2014, 53, 3904.
[11] Chen, W.; Wilde, R. G.; Seidel, D. Org. Lett. 2014, 16, 730.
/
| 〈 |
|
〉 |