

自组装金属有机纳米管模板法制备高分子纳米管
收稿日期: 2015-10-13
网络出版日期: 2015-11-13
基金资助
项目受辽宁省自然科学基金(No. 2015020246)资助.
Preparation of Polymer Nanotube Using Self-Assembled Metal Organic Nanotube as Template
Received date: 2015-10-13
Online published: 2015-11-13
Supported by
Project supported by the Natural Science Foundation of Liaoning Province of China (No. 2015020246).
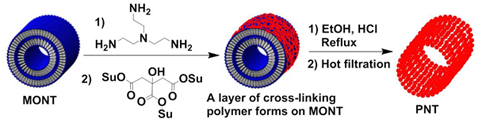
高分子纳米管(PNT)是一类具有中空管状结构的高分子纳米材料, 模板法是制备管状高分子纳米材料的有效方法. 本研究以自组装金属有机纳米管(MONT)为模板, 杈状多元胺和多元羧酸为前体分子, 利用杈状多元胺与MONT表面的铜离子配位络合, 在MONT表面上形成包覆层, 再与同样具有杈状结构的多元羧酸活化酯进行交联反应后, 去除掉内部的自组装模板, 从而制得具有良好水分散性的PNT. 利用扫描电子显微镜(SEM)、扫描透射电子显微镜(STEM)、差示扫描量热仪(DSC)、X射线衍射(XRD)和漫反射傅立叶变换红外光谱(DRIFTS)对PNT的表面形貌、组成和结构进行了表征. 结果表明, 当多元胺用量为MONT用量的0.4摩尔当量时, 交联产物的成管率最高, 达80%以上, 纳米管的长度大多为500 nm~3 μm、内径为60~100 nm、外径80~120 nm.
王雷 , 李雪 , 郑斐 , 郭玉鑫 , 张志强 , 迟海军 , 董岩 , 王翠苹 , 卢公昊 . 自组装金属有机纳米管模板法制备高分子纳米管[J]. 化学学报, 2016 , 74(3) : 259 -264 . DOI: 10.6023/A15100655
Hollow nano-material is one of the hot researches in recent years because of special optical, electrical, magnetic and catalytic properties. Polymer nanotube (PNT) is a polymer nano-material with tubular structure. Template method is an effective preparation method for tubular polymer nano-materials. In this study, we have developed a simple technique for the fabrication of polymer nanotubes by using self-assembled metal organic nanotube (MONT) as a template and multiple amine and acid as precursor molecules. An amphiphilic molecule (N-tetradecanoic glycylglycine, 1) was firstly synthesized by the coupling reaction of N-tetradecanoic acid and glycylglycine ethyl ester under the function of 1-ethyl-3-(3-dimethylamin-opropyl)carbodiimide hydrochloride (EDC·HCl), followed by a hydration process. The amphiphilic molecule 1 was successfully obtained in high yields. MONT was then prepared by the self-assembly of 1 with copper(Ⅱ) nitrate in methanol. A solution of copper(Ⅱ) nitrate in water was slowly added into a solution of 1 in methanol. The mixed solution was stirred for 24 h at room temperature and MONT was obtained by filtration, washing with water and freeze-dry. And finally, template reactions were carried out as follows: a certain amount of MONT was dispersed in tetrahydrofuran (THF), and then the multiple amine was added to complex with copper ions on the surface of MONT. The mixed solution was stirred for 3 h at room temperature and a coated layer formed on the surface of MONT. The coated layer on the nanotube surface was further cross-linked by an activated ester of citric acid. Finally, the self-assembled template was removed by hot filtration and the PNTs with good dispersibility in water were obtained. The surface topography, composition and structure of PNTs were characterized by scanning electron microscope (SEM), scanning transmission electron microscopy (STEM), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). The results showed that up to 80% cross-linked products form PNTs when the amount of multiple amines is 0.4 molar equivalent of MONT. The lengths of PNTs are about 500 nm~3 μm, inner diameters are 60~100 nm and outer diameters are 80~120 nm.

Key words: polymer nanotube; template; metal organic nanotube; self-assembly
[1] Fu, G. D.; Li, G. L.; Neoh, K. G.; Kang, E. T. Prog. Polym. Sci. 2011, 36, 127.
[2] Yin, Z.; Zheng, Q. Adv. Energy Mater. 2012, 2, 179.
[3] Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Chem. Rev. 2015, 115, 7196.
[4] Yao, Y.; Xue, M.; Chen, J.; Zhang, M.; Huang, F. J. Am. Chem. Soc. 2012, 134, 15712.
[5] Yu, G.; Ma, Y.; Han, C.; Yao, Y.; Tang, G.; Mao, Z.; Gao, C.; Huang, F. J. Am. Chem. Soc. 2013, 135, 10310.
[6] Dong, S.; Zheng, B.; Xu, D.; Yan, X.; Zhang, M.; Huang, F. Adv. Mater. 2012, 24, 3191.
[7] Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. Angew. Chem. 2011, 123, 1945.
[8] Xue, M.; Hu, S.-Z.; Chen, C.-F. Acta Chim. Sinica 2012, 70, 1697. (薛敏, 胡树振, 陈传峰, 化学学报, 2012, 70, 1697.)
[9] Thorkelsson, K.; Bai, P.; Xu, T. Nano Today 2015, 10, 48.
[10] Stupp, S. I.; Palmer, L. C. Chem. Mater. 2014, 26, 507.
[11] Chapman, R.; Danial, M.; Koh, M. L.; Jolliffe, K. A.; Perrier, S. Chem. Soc. Rev. 2012, 41, 6023.
[12] Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Chem. Rev. 2012, 112, 3959.
[13] Liu, Y.; Goebl, J.; Yin, Y. Chem. Soc. Rev. 2013, 42, 2610.
[14] Yang, X.; Tang, H.; Cao, K.; Song, H.; Sheng, W.; Wu, Q. J. Mater. Chem. 2011, 21, 6122.
[15] Leenaars, A. F. M.; Keizer, K.; Burggraaf, A. J. J. Mater. Sci. 1984, 19, 1077.
[16] Meng, X.-X.; Yang, N.-T.; Tan, X.-Y.; Guo, H. Acta Chim. Sinica 2007, 65, 871. (孟秀霞, 杨乃涛, 谭小耀, 郭红, 化学学报, 2007, 65, 871.)
[17] Martin, C. R. Science 1994, 266, 1966.
[18] Steinhart, M.; Wendorff, J.; Greiner, A.; Wehrspohn, R.; Nielsch, K.; Schilling, J.; Choi, J.; Gosele, U. Science 2002, 296, 1997.
[19] Zhang, J.; Li, C. M. Chem. Soc. Rev. 2012, 41, 7016.
[20] Wei, Y.; Sun, D.; Yuan, D.; Liu, Y.; Zhao, Y.; Li, X.; Wang, S.; Dou, J. M.; Wang, X. P.; Hao, A. Y.; Sun, D. F. Chem. Sci. 2012, 3, 2282.
[21] Panda, T.; Kundu, T.; Banerjee, R. Chem. Commun. 2012, 48, 5464.
[22] Shimizu, T.; Masuda, M.; Minamikawa, H. Chem. Rev. 2005, 105, 1401.
[23] Shimizu, T. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 2601.
[24] Shimizu, T.; Minamikawa, H.; Kogiso, M.; Aoyagi, M.; Kameta, N.; Ding, W.; Masuda, M. Polym. J. 2014, 46, 858.
[25] Kameta, N. J. Inclusion Phenom. Macrocyclic Chem. 2014, 79, 1.
[26] Kogiso, M.; Zhou, Y.; Shimizu, T. Adv. Mater. 2007, 19, 242.
[27] Aida, T.; Meijer, E. W.; Stupp, S. I. Science 2012, 335, 813.
[28] Lee, J.; Kim, S. M.; Lee, I. S. Nano Today 2014, 9, 631.
[29] Gao, Z.-Z.; Tong, H.; Chen, J.-H.; Yue, S.-H.; Bai, W.-L.; Zhang, X.-G.; Pan, Y.-F.; Shi, M.; Song, Y.-X. Acta Chim. Sinica 2014, 72, 1175. (高珍珍, 佟浩, 陈建慧, 岳世鸿, 白文龙, 张校刚, 潘燕飞, 石明, 宋玉翔, 化学学报, 2014, 72, 1175.)
[30] Guix, M.; Mayorga-Martinez, C. C.; Merkoci, A. Chem. Rev. 2014, 114, 6285.
[31] Mathews, A. S.; Kim, I.; Ha, C. S. Macromol. Res. 2007, 15, 114.
/
| 〈 |
|
〉 |