

取代基效应对喹啉腈AIE荧光性能的研究
收稿日期: 2016-01-03
网络出版日期: 2016-03-03
基金资助
项目受国家自然科学基金(No. 21325625, 21476076)资助.
Substituent Effect on Quinoline-Malononitrile AIE Fluorescent Properties
Received date: 2016-01-03
Online published: 2016-03-03
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21325625, 21476076).
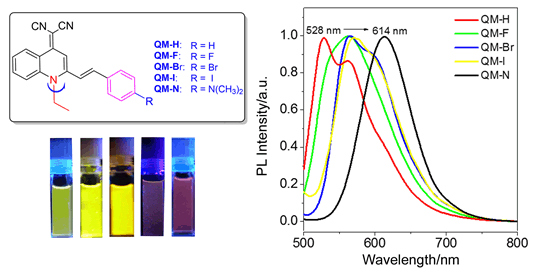
吡喃腈(DCM)类化合物作为传统的荧光染料, 其本身具有聚集荧光淬灭(Aggregation caused quenching, ACQ)的现象. 通过对吡喃腈母体进行结构修饰, 合成了一类具有长波长、聚集诱导发光(Aggregation-induced emission, AIE)的喹啉腈(QM)衍生物, 该类化合物在良溶剂中呈现弱荧光, 但在聚集态和固态时荧光增强. 并进一步研究了取代基效应对这类AIE化合物荧光性能的影响, 卤素原子的引入使得聚集态荧光基本保持在561~571 nm之间, 但荧光量子效率变化很大, QM-Br的ΦF值最大(13.9%), QM-F次之(8.7%), QM-I最小(3.4%). 给电子单元可与QM母体单元形成D-π-A结构, 其增强的推拉电子效应延长了聚集态波长, 同时能级带隙变窄. 取代基效应的研究有助于理解AIE化合物聚集微环境的变化, 为进一步发展近红外AIE荧光染料及其生物应用打下良好的基础.
夏志清 , 邵安东 , 李强 , 朱世琴 , 朱为宏 . 取代基效应对喹啉腈AIE荧光性能的研究[J]. 化学学报, 2016 , 74(4) : 351 -355 . DOI: 10.6023/A16010001
As well-known, traditional luminescent dyes such as dicyanomethylene-4H-pyran (DCM) luminogens used in biological diagnosis and therapy still exit several limitations due to their inherent molecular structures. One of the most notorious phenomena is "aggregation caused quenching" (ACQ), namely that the fluorescence can be easily observed in dilute solution, but quenched in high concentration or aggregated state. Therefore, how to understand the aggregation environment formed by dye molecules and further utilize the aggregate itself as a potential pattern for biomedical application is highly desirable. Since the intriguing discovery of the aggregation-induced emission (AIE) phenomenon, much effort has been paid to exploration of AIE systems and their applications. These AIE chromophores exhibit highly bright fluorescence when aggregated, and weak fluorescence when dissolved in solution, making them beneficial for improving the sensitivity of biosensors and bioimaging in situ or in vivo. Herein we set out to construct a novel AIE-active quinoline-malononitrile (QM) building block, by merely replacing the oxygen atom in DCM moiety with N-ethyl group, thoroughly solving the fluorescence quenching problems of DCM derivatives in aggregation. Five QM derivatives (QM-H, QM-F, QM-Br, QM-I and QM-N) with different substituent groups have been successfully synthesized by Knoevenagel reaction, extending the AIE wavelength from 528 to 614 nm in the aggregated state. A series of experiments were performed to examine the photoluminescence properties of QM-H, QM-F, QM-Br, QM-I and QM-N. As expected, all these AIE-active compounds show weak or no fluorescence in molecular state when dissolved in THF solution, but enhanced emission in solid or aggregate state along with an increasing volume fraction of water in tetrahydrofuran/water (THF/H2O) mixtures. Moreover, their AIE-active fluorescent properties are dependent upon the different aggregated microenvironment affected by substituent groups of QM derivatives. Notably, the halogen atoms of QM-F, QM-Br and QM-I play important role in AIE quantum yield, while introducing electron donor group shifts the solid fluorescence of QM-N into red emission. The substituent effect of QM derivatives with excellent AIE properties can provide a platform to develop NIR AIE materials.

[1] Guo, Z. Q.; Zhu, W. H.; Tian, H. Chem. Commun. 2012, 48, 6073.
[2] Tang, C. W.; VanSlyke, S. A.; Chen, C. H. J. Appl. Phys. 1989, 65, 3610.
[3] (a) Chen, C. H. Chem. Mater. 2004, 16, 4389.
(b) Zhong, H. L.; Lai, H.; Fang, Q. J. Phys. Chem. C 2011, 115, 2423.
[4] Luo, J. D.; Xie, Z. L.; Lam, J. W. Y.; Cheng, L.; Chen, H. Y.; Qiu, C. F.; Kwok, H. S.; Zhan, X. W.; Liu, Y. Q.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
[5] (a) Kwok, R. T. K.; Leung, C. W. T.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2015, 44, 4228.
(b) Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718.
(c) Guo, Z. Q.; Shao, A. D.; Zhu, W. H. J. Mater. Chem. C 2016, DOI: 10. 1039/C5TC03369A.
[6] (a) Zhang, X. Q.; Zhang, X. Y.; Yang, B.; Zhang, Y. L.; Wei, Y. ACS Appl. Mater. Interfaces 2014, 6, 3600.
(b) Wang, S.; Zhu, Z.; Wei, D. Q.; Yang, C. L. J. Mater. Chem. C 2015, 3, 11902.
[7] Yao, L.; Zhang, S. T.; Wang, R.; Li, W. J.; Shen, F. Z.; Yang, B.; Ma, Y. G. Angew. Chem., Int. Ed. 2014, 53, 2119.
[8] (a) Hu, F.; Huang, Y. Y.; Zhang, G. X.; Zhao, R.; Yang, H.; Zhang, D. Q. Anal. Chem. 2014, 86, 7987.
(b) Xun, Z. Q.; Tang, H. Y.; Zeng, Y.; Chen, J. P.; Yu, T. J.; Zhang, X. H.; Li, Y. Acta Chim. Sinica 2015, 73, 819. (寻知庆, 唐海云, 曾毅, 陈金平, 于天君, 张小辉, 李嫕, 化学学报, 2015, 73, 819.)
(c) Li, Y. D.; Zhang, H.; Wang, X. C.; Wang, F.; Xia, Y. J. Acta Chim., Sinica 2015, 73, 1055. (李昱达, 张恒, 王迅昶, 汪锋, 夏养君, 化学学报, 2015, 73, 1055.)
[9] Lu, H. G.; Zheng, Y. D.; Zhao, X. W.; Wang, L. J.; Ma, S. Q.; Han, X. Q.; Xu, B.; Tian, W. J.; Gao, H. Angew. Chem. Int. Ed. 2016, 55, 155.
[10] Chi, Z. G.; Zhang, X. Q.; Xu, B. J.; Zhou, X.; Ma, C. P.; Zhang, Y.; Liu, S. W.; Xu, J. R. Chem. Soc. Rev. 2012, 41, 3878.
[11] Huang, J.; Jiang, Y. B.; Yang, J.; Tang, R. L.; Xie, N.; Li, Q. Q.; Kwok, H. S.; Tang, B. Z.; Li, Z. J. Mater. Chem. C 2014, 2, 2028.
[12] Zhang, Y. P.; Li, D. D.; Li, Y.; Yu, J. H. Chem. Sci. 2014, 5, 2710.
[13] (a) Zhang, S.; Qin, A. J.; Sun, J. Z.; Tang, B. Z. Prog. Chem. 2011, 23, 623. (张双, 秦安军, 孙景志, 唐本忠, 化学进展, 2011, 23, 623.)
(b) Zhao, G. S.; Shi, C. X.; Guo, Z. Q.; Zhu, W. H.; Zhu, S. Q. Chin. J. Org. Chem. 2012, 32, 1620. (赵国生, 史川兴, 郭志前, 朱为宏, 朱世琴, 有机化学, 2012, 32, 1620.)
(c) Liu, P.; Chen, D. D.; Feng, X.; Shi, J. B.; Tong, B.; Dong, Y. P. Imag. Sci. Photochem. 2015, 33, 441 (in Chinese). (刘派, 陈笛笛, 冯霄, 石建兵, 佟斌, 董宇平, 影像科学与光化学, 2015, 33, 441.)
(d) Yu, H. B.; Li, H. L.; Zhang, X. F.; Xiao, Y.; Fang, P. J.; Lü, C. J.; Hou, W. Acta Chim. Sinica 2015, 73, 450. (于海波, 李红玲, 张新富, 肖义, 方沛菊, 吕春娇, 侯伟, 化学学报, 2015, 73, 450.)
[14] Shi, C. X.; Guo, Z. Q.; Yan, Y. L.; Zhu, S. Q.; Xie, Y. S.; Zhao, Y. S.; Zhu, W. H.; Tian, H. ACS Appl. Mater. Interfaces 2012, 5, 192.
[15] Shao, A. D.; Guo, Z. Q.; Zhu, S. J.; Zhu, S. Q.; Shi, P.; Tian, H.; Zhu, W. H. Chem. Sci. 2014, 5, 1383.
[16] Shao, A. D.; Xie, Y. S.; Zhu, S. J.; Guo, Z. Q.; Zhu, S. Q.; Guo, J.; James, T. D.; Tian, H.; Zhu, W. H. Angew. Chem., Int. Ed. 2015, 54, 7275.
[17] Yuan, W. Z.; Yu, Z.; Lu, P.; Deng, C.; Lam, J. W. Y.; Wang, Z.; Chen, E.; Ma, Y.; Tang, B. Z. J. Mater. Chem. 2012, 22, 3323.
[18] Shen, X. Y.; Wang, Y. J.; Zhao, E. G.; Yuan, W. Z.; Liu, Y.; Lu, P.; Qin, A. J.; Ma, Y. G.; Sun, J. Z.; Tang, B. Z. J. Phys. Chem. C 2013, 117, 7334.
/
| 〈 |
|
〉 |