

吲哚生物碱(+)-Alsmaphorazine D的不对称全合成及其绝对构型更正
收稿日期: 2016-02-25
网络出版日期: 2016-04-08
基金资助
项目受国家自然科学基金(No. 21472167)及浙江省杰出青年基金(No. LR16B020001)资助.
Asymmetric Total Synthesis and Absolute Configuration Reassignment of Indole Alkaloid (+)-Alsmaphorazine D
Received date: 2016-02-25
Online published: 2016-04-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21472167) and the Zhejiang Natural Science Fund for Distinguished Young Scholars (No. LR16B020001).
余宽 , 高北岭 , 丁寒锋 . 吲哚生物碱(+)-Alsmaphorazine D的不对称全合成及其绝对构型更正[J]. 化学学报, 2016 , 74(5) : 410 -414 . DOI: 10.6023/A16020102
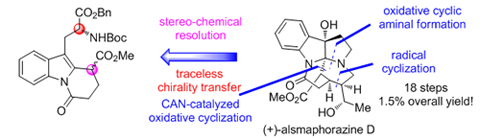
The first asymmetric total synthesis of (+)-alsmaphorazine D has been achieved through a traceless chirality transfer strategy, which also enabled absolute configuration reassignment of the natural product. Key steps of this efficient approach entail a catalytic oxidative cyclization [To a solution of indoline ester 8 (5.82 g, 10 mmol) in AcOH (100 mL) were added CAN (550 mg, 1 mmol) and NaOAc (1.64 g, 20 mmol). The reaction vessel was exposed to air through a CaCl2 tube. The resulting mixture was stirred at 110 ℃ for 12 h before it was concentrated in vacuo. The residue was diluted with H2O, neutralized with NaHCO3 (sat. aq.) and extracted with EtOAc. The combined organic layers were washed with brine, dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexanes/EtOAc, V:V=4:1) afforded δ-lactamindole 7 (4.34 g, 75%) as a white amorphous solid], a diastereoselective oxidative cyclic aminal formation [To a stirred solution of mono-ester 13 (5.20 g, 10 mmol, dr=1:1) in acetone (100 mL) at 0 ℃ was added NaHCO3 (100 mL, sat. aq.). The resulting mixture was stirred for 0.5 h before it was added oxone (12.28 g, 20 mmol) slowly. The reaction mixture was stirred at 0 ℃ for an additional 2 h before it was diluted with H2O. The aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexanes/EtOAc, V:V=3:1) afforded pyrroloindole 6' (1.61 g, 71%) as a white amorphous solid] and an intramolecular radical cyclization [To a stirred solution of ent-5 (250 mg, 0.47 mmol) in benzene (5 mL) at 80 ℃ were added n-Bu3SnH (152 μL, 0.56 mmol) and AIBN (5 μL, 0.047 mmol). The resulting mixture was stirred for 0.5 h before it was concentrated in vacuo. Flash column chromatography (silica gel, hexanes/EtOAc, V:V=1:1) afforded C(16)-epi-ent-4 (213 mg, 72%) as a colorless oil.].
[1] (a) Marini-Bettolo, G. B.; Nicoletti, M.; Messana, I.; Patamia, M.; Galeffi, C.; Oguakwa, J. U.; Portalone, G.; Vaciago, A. Tetrahedron 1983, 39, 323.
(b) Ghedira, K.; Zeches-Hanrot, M.; Richard, B.; Massiot, G.; Le Men-Olivier, L.; Sevenet, T.; Goh, S. H. Phytochemistry 1988, 27, 3955.
(c) Cai, X. H.; Du, Z. Z.; Luo, X. D. Org. Lett. 2007, 9, 1817.
[2] (a) Kamarajan, P.; Sekar, N.; Mathuram, V.; Govindasamy, S. Biochem. Int. 1991, 25, 491.
(b) Saraswathi, V.; Subramanian, S.; Ramamoorthy, N.; Mathuram, V.; Govindasamy, S. Med. Sci. Res. 1997, 25, 167.
(c) Husain, K.; Jantan, I.; Kamaruddin, N.; Said, I. M.; Aimi, N.; Takayama, H. Phytochemistry 2001, 57, 603.
(d) Khan, M. R.; Omoloso, A. D.; Kihara, M. Fitoterapia 2003, 74, 736.
(e) Gupta, R. S.; Bhatnager, A. K.; Joshi, Y. C.; Sharma, M. C.; Khushalani, V.; Kachhawa, J. B. S. Pharmacology 2005, 75, 57.
(f) Jagetia, G. C.; Baliga, M. S. Phytother. Res. 2006, 20, 103.
[3] For reviews, see: (a) Liu, J.-L. Chin. J. Org. Chem. 2003, 23, 784. (刘建利, 有机化学, 2003, 23, 784).
(b) Zhou, H.; Liu, J.; Wang, C. Chin. J. Org. Chem. 2010, 30, 1305. (周华凤, 刘建利, 王翠玲, 有机化学, 2010, 30, 1305).
(c) Wang, X.; Liu, J.; Huang, X.; Wang, C. Chin. J. Org. Chem. 2012, 32, 420. (王学军, 刘建利, 黄新炜, 王翠玲, 有机化学, 2012, 32, 420).
(d) Li, S.; Han, J.; Li, A. Acta Chim. Sinica 2013, 71, 295. (李森, 韩静, 李昂, 化学学报, 2013, 71, 295).
[4] (a) Koyama, K.; Hirasawa, Y.; Nugroho, A. E.; Kaneda, T.; Hoe, T. C.; Chan, K.-L.; Morita, H. Tetrahedron 2012, 68, 1502. For the isolation of alsmaphorazines A and B, see:
(b) Koyama, K.; Hirasawa, Y.; Nugroho, A. E.; Hosoya, T.; Hoe, T. C.; Chan, K.-L.; Morita, H. Org. Lett. 2010, 12, 4188.
[5] (a) Zhu, C.; Liu, Z.; Chen, G.; Zhang, K.; Ding, H. Angew. Chem., Int. Ed. 2015, 54, 879. For the total synthesis of (±)-alsmaphorazine B, see:
(b) Hong, A. Y.; Vanderwal, C. D. J. Am. Chem. Soc. 2015, 137, 7306.
[6] (a) Liu, X.; Cook, J. M. Org. Lett. 2001, 3, 4023.
(b) Liao, X.; Zhou, H.; Wearing, X. Z.; Ma, J.; Cook, J. M. Org. Lett. 2005, 7, 3501.
(c) Yin, W.; Kabir, M. S.; Wang, Z.; Rallapalli, S. K.; Ma, J.; Cook, J. M. J. Org. Chem. 2010, 75, 3339.
(d) Edwankar, R. V.; Edwankar, C. R.; Deschamps, J.; Cook, J. M. Org. Lett. 2011, 13, 5216.
[7] (a) Kamenecka, T. M.; Danishefsky, S. J. Chem. Eur. J. 2001, 7, 41.
(b) Trzupek, J. D.; Lee, D.; Crowley, B. M.; Marathias, V. M.; Danishefsky, S. J. J. Am. Chem. Soc. 2010, 132, 8506.
[8] (a) Peng, Q.-L.; Luo, S.-P.; Xia, X.-E.; Liu, L.-X.; Huang, P.-Q. Chem. Commun. 2014, 50, 1986.
(b) Mao, Z.-Y.; Geng, H.; Zhang, T.-T.; Ruan, Y.-P.; Ye, J.-L.; Huang, P.-Q. Org. Chem. Front. 2016, 3, 24.
[9] For selected examples, see: (a) Kamenecka, T. M.; Danishefsky, S. J. Angew. Chem., Int. Ed. 1998, 37, 2995.
(b) Baran, P. S.; Guerrero, C. A.; Corey, E. J. J. Am. Chem. Soc. 2003, 125, 5628.
(c) Umehara, A.; Ueda, H.; Tokuyama, H. Org. Lett. 2014, 16, 2526.
[10] (a) Snider, B. B. Chem. Rev. 1996, 96, 339.
(b) Tsai, A.-I.; Lin, C.-H.; Chuang, C.-P. Heterocycles 2005, 65, 2381.
[11] For the preparation, see: (a) Ma, C.; Liu, X.; Li, X.; Flippen-Anderson, J.; Yu, S.; Cook, J. M. J. Org. Chem. 2001, 66, 4525.
(b) Zhou, H.; Liao, X.; Yin, W.; Ma, J.; Cook, J. M. J. Org. Chem. 2006, 71, 251.
[12] For reviews, see: Repka, L. M.; Reisman, S. E. J. Org. Chem. 2013, 78, 12314. For selected examples, see:
(b) Savige, W. E. Aust. J. Chem. 1975, 28, 2275.
(c) Nakagawa, M.; Hino, T. Heterocycles 1977, 6, 1675.
(d) Nakagawa, M.; Kato, S.; Kataoka, S.; Hino, T. J. Am. Chem. Soc. 1979, 101, 3136.
(e) Movassaghi, M.; Schmidt, M. A. Angew. Chem., Int. Ed. 2007, 46, 3725.
(f) Furst, L.; Narayanam, J. M. R.; Stephenson, C. R. J. Angew. Chem., Int. Ed. 2011, 50, 9655.
(g) Kim, J.; Movassaghi, M. J. Am. Chem. Soc. 2011, 133, 14940.
(h) Sun, Y.; Li, R.; Zhang, W.; Li, A. Angew. Chem., Int. Ed. 2013, 52, 9201.
[13] CCDC 1445009 (6a') contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam. ac.uk/data_request/cif.
[14] For selected examples, see: (a) Schkeryantz, J. M.; Woo, J. C. G.; Danishefsky, S. J. J. Am. Chem. Soc. 1995, 117, 7025.
(b) Schkeryantz, J. M.; Woo, J. C. G.; Siliphaivanh, P.; Depew, K. M.; Danishefsky, S. J. J. Am. Chem. Soc. 1999, 121, 11964.
(c) Zhao, L.; May, J. P.; Huang, J.; Perrin, D. M. Org. Lett. 2012, 14, 90.
[15] (a) Boto, A.; HernÁndez, R.; SuÁrez, E. Tetrahedron Lett. 1999, 40, 5945.
(b) Boto, A.; HernÁndez, R.; SuÁrez, E. Tetrahedron Lett. 2000, 41, 2495.
(c) Boto, A.; HernÁndez, R.; SuÁrez, E. J. Org. Chem. 2000, 65, 4930.
[16] See SI for the detailed characterization of compound 16.
[17] (a) Evans, D. A.; Fu, G. C.; Hoveyda, A. H. J. Am. Chem. Soc. 1988, 110, 6917.
(b) Burgess, K.; van der Donk, W. A.; Westcott, S. A.; Marder, T. B.; Baker, R. T.; Calabrese, J. C. J. Am. Chem. Soc. 1992, 114, 9350.
/
| 〈 |
|
〉 |