

MoO3/C-N复合材料的制备条件对其结构及催化性能的影响
收稿日期: 2016-01-18
网络出版日期: 2016-04-26
基金资助
项目受中央高校基本科研业务费(GK200902008)资助.
Influence of Preparation Conditions of MoO3/C-N Hybrid Materials on Its Structure and Catalytic Performance
Received date: 2016-01-18
Online published: 2016-04-26
Supported by
Project supported by the Fundamental Research Funds for the Central Universities (Granted Number GK200902008).
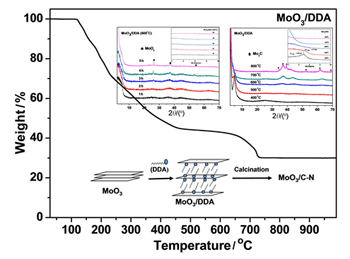
利用直接热插入法制备了MoO3/十二烷基胺(DDA)插层复合材料并对其进行煅烧处理制备了MoO3/C-N复合材料. 研究了不同的煅烧条件对该复合材料的结构、组分及形貌的影响, 结果表明在400~800 ℃之间进行煅烧, MoO3/C-N复合材料的晶态结构发生了有序-无序-有序的变化, 部分Mo的价态由+6降到+4或+2. 当煅烧温度低于600 ℃时, 主要是MoO3层间十二烷基胺的碳化反应, MoO3的层状结构仍然存在, 但层间距变小. 当煅烧温度高于600 ℃时, MoO3与C发生氧化还原反应生成Mo2C, MoO3的层状结构被破坏. 在600 ℃煅烧处理时, 随着煅烧时间的延长, 有MoO2晶体产生. 煅烧升温速率对复合材料的结构影响不大. 将不同煅烧条件制备的MoO3/C-N复合材料应用于苯甲醇制备苯甲醛的催化研究, 结果发现600 ℃煅烧2 h处理得到的MoO3/C-N复合材料的催化效果最好, 产物选择性单一, 苯甲醛产率达30%, 比未插层的MoO3(8%)提高了近4倍, 且该催化剂可多次重复使用.
张婷 , 蔡雪刁 , 刘娜 , 许春丽 . MoO3/C-N复合材料的制备条件对其结构及催化性能的影响[J]. 化学学报, 2016 , 74(5) : 441 -449 . DOI: 10.6023/A16010036
Molybdenum oxide (MoO3)/dodecylamine (DDA) intercalated materials were synthesized via direct thermal treatment followed by calcination to give MoO3/C-N hybrid materials. These prepared intercalated materials were characterized by powder X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy to investigate the influences of the calcination conditions, such as calcination temperature, calcination heating rate and calcination time, on the structure and composition of these materials. The results exhibited the order-disorder-order changes of the crystal structure during the calcination temperature from 400 ℃ to 800 ℃. Meanwhile, the valence of some Mo was reduced from +6 to +4 or +2. XRD patterns showed that calcination heating rate had almost no effect on the composite structure. Crystal MoO2 was produced with the increase of calcination time at 600 ℃ in N2 atmosphere. Crystal Mo2C was formed and the crystalline became regular with the increase of calcination temperature when the calcination temperature was higher than 600 ℃. SEM and TEM images clearly showed that molybdenum oxide layers were kept with the reducing of interlayer spacing as the calcination temperature below 600 ℃. With the calcination temperature rising up to 800 ℃, the carbonization effect of carbonaceous molecules and the enormous loss of gas molecules made the layer structure collapsed. In addition, the carbon and nitrogen elements were detected on the surface of molybdenum oxide. MoO3/C-N hybrid materials were used as catalyst for the oxidation of benzyl alcohol. The results showed that the structure and composition of the materials have a certain effect on the catalytic yield and the selectivity. The MoO3/C-N hybrid materials formed at calcination of 600 ℃ in 2 h was found to catalyze benzyl alcohol to benzaldehyde efficiently with high selectivity and relative stability. The yield of oxidation of benzyl alcohol to benzaldehyde in 3 h was up to 30% with a high selectivity retention, which was nearly 4 times compared with that of the pristine MoO3. The MoO3/C-N hybrid materials used as catalyst can be recycled several times with high selectivity.

[1] Faughnan, B. W.; Crandall, R. S. Appl. Phys. Lett. 1977, 31, 834.
[2] Yao, J. N.; Loo, B. H.; Hashimoto, K.; Fujishima, A. J. Electroanal. Chem. 1990, 290, 263.
[3] Yao, J. N.; Yang, Y. A.; Loo, B. H. J. Phys. Chem. B 1998, 102, 1856.
[4] Balendhran, S.; Deng, J.; Ou, J. Z.; Walia, S.; Scott, J.; Tang, J.; Wang, K. L.; Field, M. R.; Russo, S.; Zhuiykov, S.; Strano, M. S.; Medhekar, N.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Adv. Mater. 2013, 25, 109.
[5] Campanel, L.; Pistoia, G. J. Electrochem. Soc. 1971, 118, 1905.
[6] Zhang, Z.; Yang, R. Y.; Umar, A.; Gao, Y. S.; Wang, J. Y.; Lu, P.; Guo, Z. H.; Huang, L.; Zhou, T. T.; Wang, Q. Adv. Mater. Sci. 2014, 6(10), 2159.
[7] Brookes, C.; Wells, P. P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D. J.; Bowker, M. ACS Catal. 2014, 4(1), 243.
[8] Shuwa, S. M.; Al-Hajri, R. S.; Jibril, B. Y.; Al-Waheibi, Y. M. Electron. Mater. Lett. 2015, 11(2), 252.
[9] Meyer, J.; Hamwi, S.; Kroger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Adv. Mater. 2012, 24, 5408.
[10] Alsaif, M. M. Y. A.; Balendhran, S.; Field, M. R.; Latham, K.; Wlodarski, W.; Ou, J. Z.; Kalantar-zadeh, K. Sens. Actuators, B: Chem. 2014, 192, 196.
[11] Suzuki, T.; Yamazaki, T.; Koukitu, A.; Maeda, M.; Seki, H.; Takahashi, K. J. Mater. Sci. Lett. 1988, 7, 926.
[12] Kamiya, S.; Tsuda, D.; Miura, K.; Sasaki, N. Wear 2004, 257, 1133.
[13] Sha, X. W.; Chen, L.; Cooper, A. C.; Pez, G. P.; Cheng, H. S. J. Phys. Chem. C 2009, 113, 11399.
[14] Dong, Y. F.; Xua, X. M.; Li, S.; Han, C. H.; Zhao, K. N.; Zhang, L.; Niu, C. J.; Huang, Z.; Mai, L. Q. Nano Energy 2015, 15, 145.
[15] Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Sens. Actuators, B 2008, 128, 512.
[16] Yao, D. D.; Ou, J. Z.; Latham, K.; Zhuiykov, S.; O’Mullane, A. P.; Kalantar-zadeh, K. Cryst. Growth Des. 2012, 12, 1865.
[17] Gesheva, K. A.; Ivanova, T. M.; Bodurov, G. Prog. Org. Coat. 2012, 74, 635.
[18] Mai, L. Q.; Hu, B.; Chen, W.; Qi, Y. Y.; Lao, C. S.; Yang, R. S.; Dai, Y.; Wang, Z. L. Adv. Mater. 2007, 19, 3712.
[19] Itoh, T.; Wang, J. Z.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M.; Murayama, N. Mater. Lett. 2008, 62, 3021.
[20] Itoh, T.; Matsubara, I.; Shin, W.; Izu, N.; Nishibori, M. Mater. Chem. Phys. 2008, 110, 115.
[21] Murugana, A. V.; Viswanath, A. K. J. Appl. Phys. 2006, 100, 074319.
[22] Afsharpour, M.; Mahjoub, A. R.; Amini, M. M. J. Inorg. Organomet. Polym. 2009, 19, 298.
[23] Afsharpour, M.; Mahjoub, A. R.; Amini, M. M.; Khodadadi, A. A. Curr. Nanosci. 2010, 6, 82.
[24] Ji, H. M.; Liu, X. L.; Liu, Z. J.; Yan, B.; Chen, L.; Xie, Y. F.; Liu, C.; Hou, W. H.; Yang, G. Adv. Funct. Mater. 2015, 25, 1886.
[25] Qiu, J. Y. C.; Yang, Z. X.; Li, Y. J. Mater. Chem. A 2015, 3, 24245.
[26] Jing, Y.; Pan, Q. Y.; Cheng, Z. X.; Dong, X. W.; Xiang, Y. X. Mater. Sci. Eng. B 2007, 138, 55.
[27] Saghafi, M.; Ataie, A.; Heshmati-Manesh, S. Int. J. Refract. Met. Hard Mater. 2011, 29, 419.
[28] Ferrari, A. C.; Basko, D. M. Nature Nanotech. 2013, 46, 236.
[29] Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Nano Lett. 2007, 7, 238.
[30] Ferrari, A. C. Solid State Commun. 2007, 143, 47.
[31] Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K. S.; Basko, D. M.; Ferrari, A. C. Nano Lett. 2009, 9, 1433.
[32] Kudin, N. K.; Ozbas, B.; Schniepp, H. C.; Prud’homme, R. K.; Aksay, I. A.; Car, R. Nano Lett. 2008, 1, 36.
/
| 〈 |
|
〉 |