

有机胺盐酸盐/B(C6F5)3催化的酸与炔烃选择性加成反应研究
收稿日期: 2016-04-05
网络出版日期: 2016-04-26
基金资助
项目受国家自然科学基金(No. 21542011)和四川省教育厅(15ZA0279)资助.
Ammonium Chloride/B(C6F5)3 System Catalyzed Selective Addition of Acids to Alkynes
Received date: 2016-04-05
Online published: 2016-04-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21542011), and Scientific Research Fund of Sichuan Provincial Educational Department (15ZA0279).
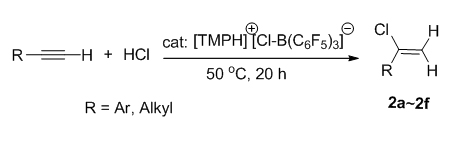
一直以来寻找直接有效的乙烯基官能化合成方法的研究备受关注. 报道了一种新型的有机胺盐酸盐/B(C6F5)3 (BCF)体系催化炔烃与氢氯酸或羧酸的加成反应方法, 可选择性地在炔烃的C(2)位氯代或羧化. 研究了在有机胺盐酸盐/BCF体系催化下, 不同取代的炔烃与无机酸HCl的氢氯化加成反应. 在2,2,4,4-四甲基哌啶盐酸盐/BCF([TMPH]+[Cl-B(C6F5)3]-)催化下, 等物质的量的炔烃和HCl反应时, 端基芳炔的C(2)位一加成产物的比例可高达90%以上, 而端基烷基炔烃的选择性较芳炔差, 叔丁基乙炔的一加成产物只占到67%. 报道了非金属催化剂路易斯酸BCF催化的炔烃与羧酸CF3COOH的烯醇酯化反应, 端基芳炔的C(2)位烯醇酯化产率可达95%以上, 而二苯基乙炔及非芳香性端基炔的反应活性较低. 首次实现了非金属催化剂FLPs参与催化的炔烃与酸的选择性氢氯化和烯醇酯化加成反应.
温志国 , 田冲 , Borzov Maxim V. , 聂万丽 . 有机胺盐酸盐/B(C6F5)3催化的酸与炔烃选择性加成反应研究[J]. 化学学报, 2016 , 74(6) : 498 -502 . DOI: 10.6023/A16040164
Development of straightforward and selective approaches to functionalize vinyl groups is an important and continuing goal. A novel convenient route to vinylhalides or enol esters by a Markovnikov regioselective addition of hydrogen chloride or carboxylic acid to the C≡C bond of alkynes in the presence of an ammonium hydrochloride/B(C6F5)3 catalytic system is reported. Thus, when treated with catalytic amounts of ammonium hydroborate ([TMPH]+[Cl-B(C6F5)3]-), equimolar mixtures of hydrogen chloride and alkynes are converted into a variety of chloroalkenes as monoadducts. The yields of the monoadducts are usually higher than 90% for terminal aromatic alkynes, while for the terminal aliphatic alkynes they are considerably lower, with the worst observed for sterically hindered tert-butylacetylene (only 67%). NMR monitoring of the reaction mixtures reveals that under ambient conditions the main by-products are the corresponding diadducts (gem-dihalides). At higher temperatures (50 ℃) for equimolar alkyne/HCl mixtures or at ambient temperature for alkyne-enriched mixtures, the diadduct formation can be nearly completely suppressed. Noteworthy, that both ammonium and borane (-ate) components of the catalytic system are essential for the conversion success. In the case of trifuoroacetic acid addition to alkynes, presence of the ammonium component is not required, with the reaction yields usually exceeding 95% for terminal aromatic alkynes and being modest to good for the aliphatic ones. The reported catalytic system presents the first example of the “metal-free” catalysts for the selective addition of acids to alkynes.

Key words: frustrated Lewis pairs; ammonium chloride; acid; alkyne; addition reaction
[1] (a) Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
(b) Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880.
(c) Spies, P.; Erker, G.; Kehr, G.; Bergander, K.; Fraeohlich, R.; Grimme, S.; Stephan, D. W. Chem. Commun. 2007, 47, 5072.
(d) Chen, D. J.; Wang, Y.; Klankermayer, J. Angew. Chem. Int. Ed. 2010, 49, 9475.
(e) Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
(f) Liu, Y.-B.; Du, H.-F. Acta Chim. Sinica 2014, 72, 771.(刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.) (g) Feng, X.-Q.; Du, H.-F. Tetrahedron Lett. 2014, 55, 6959.
[2] (a) Chase, P. A.; Welch, G. C.; Jurca, T.; Stephan, D. W. Angew Chem., Int. Ed. 2007, 119, 8196.
(b) Spies, P.; Schwendemann, S.; Lange, S.; Kehr, G.; Froehlich, R.; Erker, G. Angew Chem., Int. Ed., 2008, 120, 7654.
(c) Wang, H.; Foehlich, R.; Kehr, G.; Erker, G. Chem. Commun. 2008, 5966.
[3] (a) Stephan, D. W. J. Am. Chem. Soc. 2015, 137, 10018.
(b) Stephan, D. W. Acc. Chem. Res. 2015, 48, 306.
[4] (a) Chen, C.; Eweiner, F.; Wibbeling, B.; Fröhlich, R.; Senda, S.; Ohki, Y.; Tatsumi, K.; Grimme, S.; Kehr, G.; Erker, G. Chem. Asian J. 2010, 5, 2199.
(b) Liedtke, R.; Fröhlich, R.; Kehr, G.; Erker, G. Organometallics 2011, 30, 5222.
(c) Dierker, G.; Ugolotti, J.; Kehr, G.; Fröhlich, R.; Erker, G. Adv. Synth. Catal. 2009, 351, 1080.
[5] (a) Dureen, M. A.; Stephan, D. W. J. Am. Chem. Soc. 2009, 131, 8396.
(b) Dureen, M. A.; Brown, C. C.; Stephan, D. W. Organometallics 2010, 29, 6594.
(c) Dureen, M. A.; Brown, C. C.; Stephan, D. W. Organometallics 2010, 29, 6422.
[6] (a) Chen, C.; Kehr, G.; Fröhlich, R.; Erker, G. J. Am. Chem. Soc. 2010, 132, 13594.
(b) Chen, C.; Fröhlich, R.; Kehr, G.; Erker, G. Chem. Commun. 2010, 46, 3580.
(c) Chen, C.; Voss, T.; Fröhlich, R.; Kehr, G.; Erker, G. Org. Lett. 2011, 13, 62.
(d) Ekkert, O.; Kehr, G.; Fröhlich, R.; Erker, G. J. Am. Chem. Soc. 2011, 133, 4610.
(e) Kehr, G.; Erker, G. Chem. Commun. 2012, 48, 1839.
(f) Jiang, C. F.; Blacque, O.; Berke, H. Organometallics 2010, 29, 125.
[7] (a) Chernichenko, k.; Madarasz, A.; Papai, I.; Nieger, M.; Leskelae, M.; Repo, T. Nat. Chem. 2013, 5, 718.
[8] (a) Mahdi, T.; Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
(b) Scott, D. J.; Fuchter, M. J.; Ashley, A. E. J. Am. Chem. Soc. 2014, 136, 15813.
[9] (a) Xu, Y.-Y.; Li, Z.; Borzov, M. V.; Nie, W.-L. Prog. Chem. 2012, 24(8), 1526.(徐莹莹, 李钊, Borzov, M. V., 聂万丽, 化学进展, 2012, 24(8), 1526).
(b) Tian, C.; Borzov, M. V.; Liu, Q. CN104262374, 2015. [Chem. Abstr. 2015, 162, 219382].(聂万丽, 田冲, Borzov, M. V., 刘芹, 专利申请号CN201410415316.7, 2014).
(c) Nie, W.-L.; Tian, C.; Borzov, M. V.; Hu, X. CN104258904, 2015. [Chem. Abstr. 2015, 162, 209551].(聂万丽, 田冲, Borzov, M. V., 胡茜, 专利申请号CN201410415003.1, 2014).
(d) Nie, W.-L.; Tian, C.; Borzov, M. V.; Jiang, Y. CN104230975, 2014. [Chem. Abstr. 2014, 162, 123052].(聂万丽, 田冲, Borzov, M. V., 姜亚, 专利申请号CN201410415290.6, 2014).
(e) Hu, X.; Tian, C.; Borzov, M. V.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1025.(胡茜, 田冲, Borzov, M. V., 聂万丽, 化学学报, 2015, 73, 1025).
(f) Tian, C.; Jiang, Y.; Borzov, M. V.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1203.(田冲, 姜亚, Borzov, M. V., 聂万丽, 化学学报, 2015, 73, 1203).
[10] (a) Griesbaum, K.; Rao, R.; Leifker, G. J. Org. Chem. 1982, 47, 4975.
(b) Kropp, P. J.; Crawford, S. D. J. Org. Chem. 1994, 59, 3102.
(c) Klein, H.; Roisnel, T.; Brunean, C.; Derien, S. Chem. Commun. 2012, 48, 11032.
(d) Derien, S.; Klein, H.; Brunean, C. Angew. Chem., Int. Ed. 2015, 54, 12112.
[11] (a) Michal, R.; Youval, S. Organometallics 1983, 2, 1689.
(b) Muriel, N.; Benedicte, S.; Frauke, H.; Brunean, C.; Dixneuf, P. H. J. Organomet. Chem. 1993, 451, 133.
(c) Muriel, N.; Christian, B.; Serge, L.; Dixneuf, P. H. Tetrahedron 1993, 49(13), 2629.
(d) Olivier, L.; Pierre, H. D. J. Organomet. Chem. 1995, 488, C9.
(e) Lukas, J. G.; Jens, P.; Debasis, K. Chem. Commun. 2003, 706.
(f) Victorio, C.; Javier, F.; Jose, G. Organometallics 2011, 30, 852.
(g) Jena, R. K.; Bhattacgarjee, M. Eur. J. Org. Chem. 2015, 6734.
[12] Recently we have studied the reactivity of different kind of ammonium halide/BCF systems in respect to alkynes, and have found that the stability of a suspected σ-adduct seems to be dependent upon the nature of both the halide anion and ammonium counterion (quaternary or else). Unfortunately, the expected intermediates could not been trustworthily observed by NMR spectroscopy. This part of work is still in progress.
/
| 〈 |
|
〉 |