

收稿日期: 2016-04-21
网络出版日期: 2016-05-13
基金资助
项目受国家自然科学基金创新研究群体项目(No. 21421064)和国家重点基础研究发展计划(No. 2013CB834502)资助.
Controlled Fabrication of Two-Dimensional Organic Assemblies
Received date: 2016-04-21
Online published: 2016-05-13
Supported by
Project supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 21421064) and the National Basic Research Program of China (No. 2013CB834502).
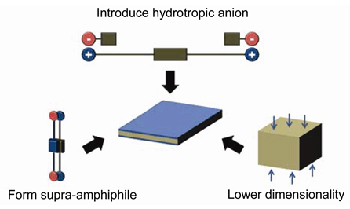
二维有机组装体是一类具有特殊形貌和性质的有序结构, 有可能带来新功能和光电子领域的潜在应用, 但如何实现二维有机组装体的可控制备是尚待解决的问题. 针对这一问题, 我们通过对构筑基元的理性设计, 调控分子间的相互作用, 发展了三种可控制备二维有机组装体的新方法: (1)利用疏水有机阴离子作为Bola型两亲分子的抗衡离子, 能够削弱亲水头基间的静电排斥作用, 从而诱导两亲分子的组装结构从一维向二维转变; (2)基于非共价键形成超两亲分子, 通过设计和控制超两亲分子的拓扑结构, 简便有效地实现二维组装体的制备; (3)通过共价修饰或引入新的非共价键, 以限制三维结构在某一方向上的生长, 从而降低三维结构的维度, 也能实现二维组装体的可控制备. 未来, 上述研究有望进一步拓展, 并实现功能二维有机组装体的构筑.
徐俊 , 王治强 , 张希 . 二维有机组装体的可控制备[J]. 化学学报, 2016 , 74(6) : 467 -471 . DOI: 10.6023/A16040200
Two-dimensional (2D) organic assemblies possess many intriguing properties such as planar structure, extended surface, flexibility and tailorability. The fabrication of 2D organic structures in a controlled manner is crucial to realize their potential functions and applications. In recent years, we have developed three methods to fabricate 2D organic assemblies through the rational design of building blocks. Firstly, hydrotropic anions will induce cationic bolaamphiphiles to form 2D assemblies, because such anions can insert their hydrophobic parts into the assemblies and weaken the electrostatic repulsion between adjacent headgroups. Secondly, the topology of supra-amphiphiles can be easily tuned because of the dynamic nature of non-covalent bonds, thus providing a facile approach for the fabrication of 2D assemblies. Thirdly, through molecular modification or introduction of new non-covalent bonds, we can lower the dimensionality of three-dimensional (3D) networks to form 2D assemblies. It is highly anticipated that these methods can be further applied for the preparation of functional 2D nano/micro materials for optoelectronics.

[1] Kimizuka, N.; Kawasaki, T.; Kunitake, T. J. Am. Chem. Soc. 1993, 115, 4387.
[2] Davis, R.; Berger, R.; Zentel, R. Adv. Mater. 2007, 19, 3878.
[3] Chen, Y.; Zhu, B.; Zhang, F.; Han, Y.; Bo, Z. Angew. Chem., Int. Ed. 2008, 47, 6015.
[4] Lee, E.; Kim, J. K.; Lee, M. Angew. Chem., Int. Ed. 2009, 48, 3657.
[5] Nam, K. T.; Shelby, S. A.; Choi, P. H.; Marciel, A. B.; Chen, R.; Tan, L.; Chu, T. K.; Mesch, R. A.; Lee, B. C.; Connolly, M. D.; Kisielowski, C.; Zuckermann, R. N. Nat. Mater. 2010, 9, 454.
[6] An, Q.; Chen, Q.; Zhu, W.; Li, Y.; Tao, C. A.; Yang, H.; Li, Z.; Wan, L.; Tian, H.; Li, G. Chem. Commun. 2010, 46, 725.
[7] Zhao, Y. S.; Fu, H.; Peng, A.; Ma, Y.; Xiao, D.; Yao, J. Adv. Mater. 2008, 20, 2859.
[8] Li, R.; Hu, W.; Liu, Y.; Zhu, D. Acc. Chem. Res. 2010, 43, 529.
[9] Li, S.; Huang, X.; Zhang, H. Acta Chim. Sinica 2015, 73, 913.(李绍周, 黄晓, 张华, 化学学报, 2015, 73, 913.)
[10] Israelachvili, J. N.; Marcelja, S.; Horn, R. G. Q. Rev. Biophys. 1980, 13, 121.
[11] Leontidis, E. Curr. Opin. Colloid. In. 2002, 7, 81.
[12] Song, B.; Wang, Z.; Chen, S.; Zhang, X.; Fu, Y.; Smet, M.; Dehaen, W. Angew. Chem., Int. Ed. 2005, 44, 4731.
[13] Yin, S.; Song, B.; Liu, G.; Wang, Z.; Zhang, X. Langmuir 2007, 23, 5936.
[14] Song, B.; Liu, G.; Xu, R.; Yin, S.; Wang, Z.; Zhang, X. Langmuir 2008, 24, 3734.
[15] Wu, G.; Verwilst, P.; Xu, J.; Xu, H.; Wang, R.; Smet, M.; Dehaen, W.; Faul, C. F. J.; Wang, Z.; Zhang, X. Langmuir 2012, 28, 5023.
[16] Wu, G.; Thomas, J.; Smet, M.; Wang, Z.; Zhang, X. Chem. Sci. 2014, 5, 3267.
[17] Wu, G.; Verwilst, P.; Liu, K.; Smet, M.; Faul, C. F. J.; Zhang, X. Chem. Sci. 2013, 4, 4486.
[18] Zhang, X.; Wang, C. Chem. Soc. Rev. 2011, 40, 94.
[19] Wang, C.; Wang, Z.; Zhang, X. Acc. Chem. Res. 2012, 45, 608.
[20] Zhang, X.; Wang, C.; Wang, Z. Sci. China-Chem. 2011, 41, 216.(张希, 王朝, 王治强, 中国科学: 化学, 2011, 41, 216.)
[21] Kimizuka, N.; Kawasaki, T.; Hirata, K.; Kunitake, T. J. Am. Chem. Soc. 1998, 120, 4094.
[22] Oda, R.; Huc, I.; Schmutz, M.; Candau, S. J.; MacKintosh, F. C. Nature 1999, 399, 566.
[23] Wang, C.; Yin, S.; Chen, S.; Xu, H.; Wang, Z.; Zhang, X. Angew. Chem., Int. Ed. 2008, 47, 9049.
[24] Zhang, X.; Chen, Z.; Wurthner, F. J. Am. Chem. Soc. 2007, 129, 4886.
[25] Liu, K.; Wang, C.; Li, Z.; Zhang, X. Angew. Chem., Int. Ed. 2011, 50, 4952.
[26] Li, F.; Song, Q.; Yang, L.; Wu, G.; Zhang, X. Chem. Commun. 2013, 49, 1808.
[27] Athikomrattanakul, U.; Promptmas, C.; Katterle, M.; Schilde, U. Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, o2154.
[28] Xu, J.; Wu, G.; Wang, Z.; Zhang, X. Chem. Sci. 2012, 3, 3227.
[29] Miyazaki, Y.; Kanbara, T.; Yamamoto, T. Tetrahedron Lett. 2002, 43, 7945.
[30] Yi, Y.; Fa, S.; Cao, W.; Zeng, L.; Wang, M.; Xu, H.; Zhang, X. Chem. Commun. 2012, 48, 7495.
/
| 〈 |
|
〉 |