

邻羟基苄醇与邻羟基苯乙烯的催化不对称[4+2]环加成反应——手性色满骨架的立体选择性构建
收稿日期: 2016-02-05
网络出版日期: 2016-06-07
基金资助
项目受国家自然科学基金(Nos.21372002and21232007),江苏省优势学科及江苏省青蓝工程项目资助.
Catalytic Asymmetric[4+2] Cycloaddition of o-Hydroxybenzyl Alcohols with o-Hydroxyl Styrenes: Diastereo- and Enantioselective Construction of Chiral Chroman Scaffold
Received date: 2016-02-05
Online published: 2016-06-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372002 and 21232007), Priority Academic Program Development of Jiangsu Higher Education Institutions and QingLan project of Jiangsu Province.
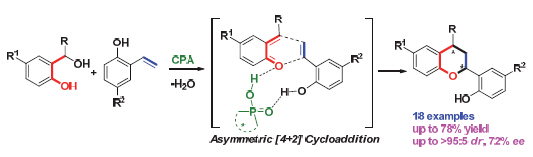
研究了手性磷酸催化下邻羟基苄醇和邻羟基苯乙烯的不对称[4+2]环加成反应,立体选择性地一步构建了手性2,4-二取代四氢色满骨架,该反应具有较高的收率、中等到较高的对映选择性和很好的非对映选择性(最高产率为78%,最高ee值为72%,dr值基本都大于95∶5).带有不同取代基的多种邻羟基苄醇和邻羟基苯乙烯均适用于该反应,电子效应对于该反应的对映选择性有一定影响,其中连有供电子基的底物具有更高的反应活性和对映选择性.由邻羟基苄醇原位生成的邻亚甲基苯醌中间体和邻羟基苯乙烯可以同时与催化剂手性磷酸形成双重氢键,对于促进反应的进行和控制该反应的对映选择性起着至关重要的作用.
吴琼 , 赵佳佳 , 孙斯兵 , 屠蔓苏 , 石枫 . 邻羟基苄醇与邻羟基苯乙烯的催化不对称[4+2]环加成反应——手性色满骨架的立体选择性构建[J]. 化学学报, 2016 , 74(7) : 576 -581 . DOI: 10.6023/A16020080
A chiral phosphoric acid-catalyzed asymmetric[4+2] cycloaddition of o-hydroxyl styrenes with o-quinone methides (o-QMs) generated in situ from o-hydroxybenzyl alcohols has been established. O-Hydroxybenzyl alcohols could transform into o-QM intermediates under the catalysis of chiral phosphoric acid (CPA), which are easily activated by CPA via hydrogen-bonding interaction. On the other hand, o-hydroxyl styrenes could also be activated by CPA via forming a hydrogen bond between the hydroxyl group of styrenes and the phosphoryl oxygen of CPA. So, by selecting o-hydroxybenzyl alcohols as precursors of dienes and o-hydroxyl styrenes as dienophiles under the catalysis of CPA, this catalytic asymmetric[4+2] cycloaddition provided an efficient strategy for constructing enantioenriched chroman framework with two stereogenic centers. A variety of substituted o-hydroxybenzyl alcohols and o-hydroxyl styrenes bearing either electron-donating or electron-withdrawing groups could be applicable to the reaction, delivering chiral chroman derivatives in high yields, considerable enantioselectivities and excellent diastereoselectivities (up to 78% yield, 72% ee, most of examples>95∶5 dr). The electronic nature of the substituents has some effect on the reaction. Namely, the electron-donating groups were beneficial to both the reactivity and the enantioselectivity. Based on the control experiments, it is suggested that the o-hydroxyl styrenes and the o-QM intermediates generated from o-hydroxybenzyl alcohols were simultaneously activated by CPA via forming double hydrogen bonds, thus facilitating the reaction in an enantioselective way. A representative procedure for the enantioselective[4+2] cycloaddition reaction is as following: 1,2-dichloroethane (1 mL) was added to the mixture of o-hydroxybenzyl alcohols (0.1 mmol), o-hydroxyl styrenes (0.12 mmol), the chiral phosphoric acid (0.005 mmol), and 3 Å molecular sieves (100 mg). After being stirred at 50 ℃ for 12 h, the reaction mixture was filtered to remove the molecular sieves, and the solid powder was washed with ethyl acetate. The resultant solution was concentrated under the reduced pressure to give the residue, which was purified through flash column chromatography on silica gel to afford the pure chiral chroman derivatives.

[1] For some examples, see: (a) Ko, H.-H.; Jin, Y.-J.; Lu, T.-M.; Chen, I.-S. Chem. Biodiversity 2013, 10, 1269;
(b) Kumar, S.; Deshpande, S.; Chandra, V.; Kitchlu, S.; Dwivedi, A.; Nayak, V. L.; Konwar, R.; Prabhakar; Yenamandra, S.; Sahu, D. P. Bioorg. Med. Chem. 2009, 17, 6832;
(c) Hafez, H. N.; Hegab, M. I.; Ahmed-Farag, I. S.; El-Gazzar, A. B. A. Bioorg. Med. Chem. Lett. 2008, 18, 4538;
(d) Poupelin, J. P.; Saint-Ruf, G.; Foussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.; Lacroix, R. Eur. J. Med. Chem. 1978, 13, 67.
[2] (a) Kumar, S.; Deshpande, S.; Chandra, V.; Kitchlu, S.; Dwivedi, A.; Nayak, V. L.; Konwar, R.; Prabhakar, Y. S.; Sahu, D. P. Bioorg. Med. Chem. 2009, 17, 6832;
(b) Sangita; Dwivedi, A.; Prathipati, P.; Ray, S. Med. Chem. Res. 2010, 19, 915.
[3] For some examples, see: (a) Tripathi, S.; Dwivedy, I.; Dhar, J. D.; Dwivedy, A.; Ray, S. Bioorg. Med. Chem. Lett. 1997, 7, 2131;
(b) Ferreira, S. B.; da Silva, F. de C.; Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080;
(c) Zhang, H.; Zhu, L.; Wang, S.; Yao, Z.-J. J. Org. Chem. 2014, 79, 7063;
(d) Yu, S.-Y.; Zhang, H.; Gao, Y.; Mo, L.; Wang, S.; Yao, Z.-J. J. Am. Chem. Soc. 2013, 135, 11402;
(e) Enders, D.; Urbanietz, G.; Hahn, R.; Raabe, G. Synthesis 2012, 44, 773.
[4] For some reviews, see: (a) van de Water, R. W.; Pettus, T. R. R. Tetrahedron 2002, 58, 5367;
(b) Pathak, T. P.; Sigman, M. S. J. Org. Chem. 2011, 76, 9210;
(c) Willis, N. J.; Bray, C. D. Chem.-Eur. J. 2012, 18, 9160;
(d) Wang, Z.; Sun, J. Synthesis 2015, 47, 3629; For some enantioselective examples, see:
(e) Alden-Danforth, E.; Scerba, M. T.; Lectka, T. Org. Lett. 2008, 10, 4951;
(f) Lv, H.; You, L.; Ye, S. Adv. Synth. Catal. 2009, 351, 2822;
(g) Pathak, T. P.; Gligorich, K. M.; Welm, B. E.; Sigman, M. S. J. Am. Chem. Soc. 2010,132, 7870;
(h) Luan, Y.; Schaus, S. E. J. Am. Chem. Soc. 2012, 134, 19965;
(i) Lv, H.; Jia, W. Q.; Sun, L. H.; Ye, S. Angew. Chem., Int. Ed. 2013, 52, 8607;
(j) Izquierdo, J.; Orue, A.; Scheidt, K. A. J. Am. Chem. Soc. 2013, 135, 10634;
(k) Wang, Z. B.; Ai, F. J.; Wang, Z.; Zhao, W. X.; Zhu, G. Y.; Lin, Z. Y.; Sun, J. W. J. Am. Chem. Soc. 2015, 137, 383.
[5] (a) Wilcke, D.; Herdtweck, E.; Bach, T. Synlett 2011, 2011, 1235;
(b) Zhao, W.; Wang, Z.; Chu, B.; Sun, J. Angew. Chem., Int. Ed. 2015, 54, 1910;
(c) Saha, S.; Alamsetti, S. K.; Schneider, C. Chem. Commun. 2015, 51, 1461.
[6] (a) El-Sepelgy, O.; Haseloff, S.; Alamsetti, S. K.; Schneider, C. Angew. Chem., Int. Ed. 2014, 53, 7923;
(b) Hsiao, C. C.; Liao, H. H.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 13258;
(c) Saha, S.; Schneider, C. Chem. Eur. J. 2015, 21, 2348;
(d) Saha, S.; Schneider, C. Org. Lett. 2015, 17, 648;
(e) Zhao, J.-J.; Zhang, Y.-C.; Xu, M.-M.; Tang, M.; Shi, F. J. Org. Chem. 2015, 80, 10016.
[7] (a) Zhao, J.-J.; Sun, S.- B.; He, S.-H.; Wu, Q.; Shi, F. Angew. Chem., Int. Ed. 2015, 54, 5460;
(b) Hsiao, C.-C.; Raja, S.; Liao, H.-H.; Atodiresei, I.; Rueping, M. Angew. Chem., Int. Ed. 2015, 54, 5762.
[8] For early examples: (a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566;
(b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356; For reviews:
(c) Akiyama, T. Chem. Rev. 2007, 107, 5744;
(d) Terada, M. Chem. Commun. 2008, 35, 4097;
(e) Terada, M. Synthesis 2010, 1929;
(f) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156;
(g) Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Chem. Rev. 2014, 114, 9047;
(h) Wu, X.; Li, M.-L.; Gong, L.-Z. Acta Chim. Sinica 2013, 71, 1091. (吴祥, 李明丽, 龚流柱, 化学学报, 2013, 71, 1091.);
(i) Wu, H.; He, Y.-P.; Shi, F. Synthesis 2015, 47, 1990; For some selected examples, see:
(j) Huang, J.-Z.; Luo, S.-W.; Gong, L.-Z. Acta Chim. Sinica 2013, 71, 879. (黄建洲, 罗时玮, 龚流柱, 化学学报, 2013, 71, 879);
(k) Duan, D.; Yin, Q.; Wang, S.; Gu, Q.; You, S. Acta Chim. Sinica 2014, 72, 1001. (段德河, 殷勤, 王守国, 顾庆, 游书力, 化学学报, 2014, 72, 1001.);
(l) Shi, L.; Ji, Y.; Huang, W.; Zhou, Y. Acta Chim. Sinica 2014, 72, 820. (时磊, 姬悦, 黄文学, 周永贵, 化学学报, 2014, 72, 820.);
(m) Lv, J.; Qin, Y.; Cheng, J.; Luo, S. Acta Chim. Sinica 2014, 72, 809. (吕健, 秦岩, 程津培, 罗三中, 化学学报, 2014, 72, 809.);
(n) Wang, S.-G.; You, S.-L. Angew. Chem., Int. Ed. 2014, 53, 2194;
(o) Wang, S.-G.; Yin, Q.; Zhuo, C.-X.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 647.
[9] (a) Chen, X.-H.; Zhang, W.-Q.; Gong, L.-Z. J. Am. Chem. Soc. 2008, 130, 5652;
(b) He, L.; Chen, X.-H.; Wang, D.-N.; Luo, S.-W.; Zhang, W.-Q.; Yu, J.; Ren, L.; Gong, L.-Z. J. Am. Chem. Soc. 2011, 133, 13504.
/
| 〈 |
|
〉 |