

CL20/DNB共晶的热感度分子动力学研究
Thermal Sensitivity of CL20/DNB Co-crystal Research via Molecular Dynamics Simulations
Received date: 2016-03-22
Online published: 2016-06-07
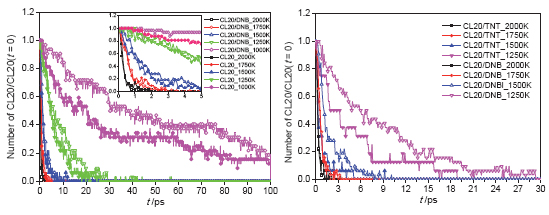
共晶技术大幅提高了六硝基六氮杂异伍兹烷(CL20)的热稳定性而且保留了CL20高爆速、高爆压等特性.为了研究CL20/1,3-硝基甲苯(DNB)共晶的热感度降低的原因,采用ReaxFF/lg反应力场模拟CL20/DNB共晶、CL20/TNT共晶、CL20单晶以及DNB单晶系统的热分解过程.研究发现: CL20/DNB共晶的热感度低于CL20/TNT共晶和CL20单晶的热感度,但高于DNB单晶的热感度.通过分析CL20单晶和CL20/DNB共晶的初始反应路径,揭示了共晶有效降低CL20热感度的机理.CL20/DNB共晶和CL20热分解具有相似的主要产物,NO2、NO3、N2、N2O2、HNO、H2O、CO2和HONO等,通过反应动力学分析得到CL20/DNB共晶和CL20的活化能.
关键词: ReaxFF/lg; 分子动力学; CL20/DNB共晶; 反应机理; 热感度
杨镇 , 薛一江 , 何远航 . CL20/DNB共晶的热感度分子动力学研究[J]. 化学学报, 2016 , 74(7) : 612 -619 . DOI: 10.6023/A16030141
Co-crystal technology has effectively improved the safety of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12- hexaazaisowurtzitane (CL20) and retained high detonation velocity, detonation pressure and other properties of CL20. To study why thermal sensitivity of CL20/1,3-dinitrobenzene (DNB) co-crystal can be effectively reduced, simulations of the pyrolysis process of CL20/DNB co-crystal, CL20/TNT co-crystal, CL20 crystal and DNB crystal system were made with ReaxFF/lg force field reactive molecular dynamics in this paper. This paper provides from atomic level detailed information on thermal decomposition processes of CL20, DNB, CL20/DNB co-crystal and CL20/TNT co-crystal such as reaction pathway, contributing to a better understanding of differences between co-crystal and pure crystal. The results show that CL20/DNB co-crystal has lower thermal sensitivity than CL20 and CL20/TNT co-crystal, but has higher thermal sensitivity than DNB, which is consistent with experimental data. Besides, the initial reaction pathways of CL20 and CL20/DNB were found, which reveals the reasons why co-crystal can effectively reduce CL20 thermal sensitivity. CL20 and CL20/DNB have similar initial reaction pathways: N—NO2 bond of CL20 molecules breaks, working as a dominant role in the initial stage of thermal decomposition under the condition of different temperatures, followed by cage skeleton structure breaking reaction. We found two reasons for the decrease rate of CL20 in CL20/DNB co-crystal decomposition: (A) The huge number of DNB molecules in the initial reaction stage prevents effective collision between CL20 and the intermediate reactants, thus lowering the speed of CL20 thermal decomposition. (B) Part of DNB molecules react with CL20 molecules and the intermediate products, producing C6H4N3O6, C6H4N4O8 and C10H10N14O16 etc., which decrease concentration of CL20. Both are explanations from microscopic mechanism on why the thermal sensitivity of CL20/DNB co-crystal is lower than that of CL20 crystal. In addition, CL20/DNB co-crystal and CL20 have similar main reactants, such as NO2, NO3, N2, N2O2, HNO, H2O, CO2, and HONO etc. Through the analysis of the reaction kinetics, we obtain activation barrier of CL20 system and CL20/DNB system. This study confirms the fact that co-crystallization is an effective way to decrease the thermal sensitivity for CL20 while retaining high detonation performance.

[1] Nielsen, A. T. US Appl. 07/253106, 1988.
[2] Nielsen, A. T.; Chafin, A. P.; Christian, S. L.; Moore, D. W.; Nadler, M. P.; Nissan, R. A.; Vanderah, D. J.; Gilardi, R. D.; George, C. F.; Flippen-Anderson, J. L. Tetrahedron 1998, 54, 11793.
[3] Simpson, R. L.; Urtiew, P. A.; Ornellas, D. L.; Moody, G. L.; Scribner, K.; Hoffman, D. M. Propellants Explos. Pyrotech. 1997, 22, 249.
[4] Ordzhonikidze, O.; Pivkina, A.; Frolov, Y.; Muravyev, N.; Monogarob, K. J. Therm. Anal. Calorim. 2011, 105, 529.
[5] Guo, C. Y.; Zhang, H. B.; Wang, X. C.; Sun, J. Mater. Rev. 2012, 26(10), 49 (in Chinese). (郭长艳, 张浩斌, 王小川, 孙杰, 材料导报, 2012, 26(10), 49.)
[6] Zhang, C. Y.; Cao, Y. F.; Li, H. Z.; Zhou, Y.; Zhou, J. H.; Gao, T.; Zhang, H. W.; Yang, Z. W.; Jiang, G. CrystEngComm 2013, 15, 4003.
[7] Bolton, O.; Matzger, A. J. Angew. Chem. Int. Ed. 2011, 50, 8960.
[8] Yang, Z. W.; Zhang, Y. L.; Li, H. Z.; Zhou, X. Q.; Nie, F. D.; Li, J. S.; Huang, H. Chin. J. Energ. Mater. 2012, 20, 674 (in Chinese). (杨宗伟, 张艳丽, 李洪珍, 周小清, 聂福德, 李金山, 黄辉, 含能材料, 2012, 20, 674.)
[9] Bolton, O.; Simke, L. R.; Pagoria, P. E.; Matzger, A. J. Cryst. Growth Des. 2012, 12, 4311.
[10] Sun, T.; Liu, Q.; Xiao, J. J.; Zhao, F.; Xiao, H. M. Acta Chim. Sinica 2014, 72, 1036 (in Chinese). (孙婷, 刘强, 肖继军, 赵峰, 肖鹤鸣, 化学学报, 2014, 72, 1036.)
[11] Wang, Y. P.; Yang, Z. W.; Li, H. Z.; Zhang, Q.; Wang, J. H.; Liu, Y. C. Propellants Explos. Pyrotech. 2014, 39, 590.
[12] Gong, X. B.; Sun, C. H.; Pang, S. P.; Zhang, J.; Li, Y. C.; Zhao, X. Q. Chinese J. Org. Chem. 2012, 32, 486 (in Chinese). (公绪滨, 孙成辉, 庞思平, 张静, 李玉川, 赵信岐, 有机化学, 2012, 32, 486.)
[13] Xu, X. J.; Xiao, J. J.; Huang, H.; Li, J. S.; Xiao, H. M. Sci. China Ser. B 2007, 50, 737.
[14] Isayev, O.; Gorb, L.; Qasim, M.; Leszczynski, J. J. Phys. Chem. B 2008, 112, 11005.
[15] Byrd, E. F. C.; Scuseria, G. E.; Chabalowski, C. F. J. Phys. Chem. B 2004, 108, 13100.
[16] Xu, X. J.; Xiao, H. M.; Ju, X. H.; Gong, X. D. Chin. J. Org. Chem. 2005, 25, 536 (in Chinese). (许晓娟, 肖鹤鸣, 居学海, 贡雪东, 有机化学, 2005, 25, 536.)
[17] Tian, Q.; Yan, G. Y.; Sun, G. G.; Huang, C. Q.; Xie, L.; Chen, B.; Huang, M.; Li, H. Z.; Liu, Y.; Wang, J. Cent. Eur. J. Energ. Mat. 2013, 10, 359.
[18] Golofit, T.; Zy?k, K. J. Therm. Anal. Calorim. 2015, 119, 1931.
[19] Liu, H.; Li, Q. K.; He, Y. H. Acta Phys. Sin. 2013, 62, 1 (in Chinese). (刘海, 李启楷, 何远航, 物理学报, 2013, 62, 1.)
[20] Liu, H.; Li, Q. K.; He, Y. H. Acta Phys. Sin. 2015, 64, 018201-1 (in Chinese). (刘海, 李启楷, 何远航, 物理学报, 2015, 64, 018201-1.)
[21] Furman, D.; Kosloff, R.; Dubnikova, F.; Zybin, S. V.; Goddard, W. A. J. Am. Chem. Soc. 2014, 136, 4192.
[22] Cheng, T.; Jaramillo-Botero, A.; Goddard, W. A.; Sun, H. J. Am. Chem. Soc. 2014, 136, 9434.
[23] Yang, Z.; He, Y. H. Acta Phys.-Chim. Sin. 2016, 32, 921 (in Chinese). (杨镇, 何远航, 物理化学学报, 2016, 32, 921.)
[24] Yang, Z.; Liu, H.; He, Y. H. Acta Phys. Chim. Sin. 2016, doi: 10.3866/PKU.WHXB201604293
[25] Diao, Z.; Zhao, Y.; Chen, B.; Duan, C. Acta Chim. Sinica 2012, 70, 2037 (in Chinese). (刁智俊, 赵跃民, 陈博, 段晨龙, 化学学报, 2012, 70, 2037.)
[26] http://lammps.sandia.gov/ (accessed Mar. 21, 2016).
[27] Gonzalez, A. C.; Larson, C. W.; McMilen, D. F.; Golden, D. M. J. Phys. Chem. 1985, 89, 4809.
[28] Fields, E.; Meyerson, S. J. Org. Chem. 1972, 37, 3861.
[29] Fang, M.; Li, Z.; Fu, Y. Chinese J. Chem. 2008, 26, 1122.
[30] Rice, B. M.; Sahu, S.; Owens, F. L. J. Mol. Struct. (Theochem). 2002, 583(1-3), 69.
[31] Shao, J. X.; Cheng, X. L.; Yang, X. D. Struct. Chem. 2006, 17, 547.
[32] Wang, G.; Gong, X.; Liu, Y.; Xiao, H. Chinese J. Chem. 2009, 27, 1669.
[33] Turcotte, R.; Vachon, M.; Kwok, Q. S. M.; Wang, R.; Jone, D. E. G. Thermochim. Acta 2005, 433(1-2), 105.
[34] Nair, U. R.; Sivabalan, R.; Gore, G. M.; Geetha, M.; Asthana, S. N.; Singh, H. Combust. Explos. Shock Waves 2005, 41, 121.
[35] Nedelko, V. V.; Chukanov; Raevskii, A. V.; Korsounskii, B. L.; Larikova, T. S.; Kolesova, O. I. Propellants Explos. Pyrotech. 2000, 25, 255.
[36] Meent, A.; Dittrich, B.; Johnas, S. K. J.; Thome, V.; Weckert, E. F. Acta Crystallogr. Sect. B: Struct. Sci. 2008, 64, 42.
[37] Wojcik, G.; Mossakowska, I.; Holband, J.; Bartkowiak, W. Acta Crystallogr. B 2002, 58, 998.
/
| 〈 |
|
〉 |