

组氨酸功能化石墨烯量子点@纳米硅负极材料的制备及电化学性能研究
收稿日期: 2016-01-27
网络出版日期: 2016-07-19
基金资助
项目受国家自然科学基金(No.21576115)资助.
Study on the Synthesis and Electrochemical Performance of Histidine-Functionalized Graphene Quantum Dots@Silicon Composite Anode Material
Received date: 2016-01-27
Online published: 2016-07-19
Supported by
Project supported by the National Natural Science Foundation of China (No. 21576115).
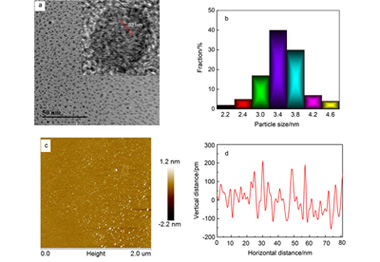
以柠檬酸和组氨酸混合物为碳源采用高温热解法制备组氨酸功能化石墨烯量子点(CH-GQD).CH-GQD是由平均尺寸仅为3.5 nm的石墨烯片组成,片的边缘含有丰富亲水基团,产品极易溶于水,具有强而稳定的荧光发射.将CH-GQD包覆于硅纳米粒子表面得到石墨烯量子点@硅复合物,以此复合物电极为负极、金属锂片为正极装配锂电池,并测试其电化学性能.研究表明,CH-GQD的引入使硅负极的电子转移阻抗下降超过14.7倍,电极与电解质之间的锂离子扩散系数提高310倍,减少了因硅与电解液分子发生副反应造成的储锂容量迅速衰减.CH-GQD@Si电池在50和1000 mA·g-1恒电流下首次放电容量分别是3325和1119 mAh·g-1.在100 mA·g-1电流密度下循环100圈放电容量仍保持1454.4 mAh·g-1.CH-GQD@Si的电池行为明显优于硅负极和柠檬酸和丙氨酸热解产生石墨烯量子点(CA-GQD)改性后的硅负极.由于CH-GQD和CA-GQD在结构上仅相差一个咪唑边缘基团,上述结果还证明咪唑基对提高复合物电极电化学性能发挥了重要作用.
孔丽娟 , 周晓燕 , 范赛英 , 李在均 , 顾志国 . 组氨酸功能化石墨烯量子点@纳米硅负极材料的制备及电化学性能研究[J]. 化学学报, 2016 , 74(7) : 620 -628 . DOI: 10.6023/A16010060
The mixture of citric acid and histidine was used as the carbon source for the preparation of histidine- functionalized graphene quantum dots via a high temperature pyrolysis (CH-GQD). The as-prepared CH-GQD is composed of graphene sheets with an average size of 3.5 nm. The edge of graphene sheets contains the rich of hydrophilic groups. The product is very soluble in water and displays strong and stable fluorescence emission. CH-GQD was coated on the surface of silica nanoparticles to obtain graphene quantum dots-silicon composite. Then, the lithium ion battery was assembled and its electrochemical performance was investigated, in which the composite electrode and metal lithium plate were used as the anode and the cathode, respectively. The results show that the introduction of CH-GQD leads to decrease of the electron transfer impedance of the silicon cathode by more than 14.7 times, increase of the lithium ion diffusion coefficient between the electrode and the electrolyte by 310 times, and reduce of storage lithium capacity fading caused by the side reactions of the silicon atoms with the electrolyte molecules. The first discharge capacity of CH-GQD@Si cell reaches 3325 mAh·g-1 at the current density of 50 mA·g-1 and 1119 mAh·g-1 at the current density of 1000 mA·g-1. The discharge capacity can remain 1454.4 mAh·g-1 at least after 100 cycles at the current density of 100 mA·g-1. The battery performance of CH-GQD@Si composite electrode is obviously better than that of pristine silicon anode and the modified silicon anode with the graphene quantum dots (CA-GQD), which was produced by the pyrolysis of citric acid and alanine. Because the difference in the structure between CH-GQD and CA-GQD only is the imidazole groups on the edge of their graphene sheets, the above result also proves that the imidazole group plays important roles to improve the electrochemical performance of the composite electrode.

[1] Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J. M. Nature. Mater. 2012, 11, 19.
[2] Tarascon, J. M.; Armand, M. Nature 2001, 414, 359.
[3] Megahed, S.; Scrosati, B. J. Power Sources 1994, 51, 79.
[4] Kasavajjula, U.; Wang, C.; Appleby, A. J. J. Power Sources 2007, 163, 1003.
[5] Luo, F.; Zheng, J.-Y.; Chu, G.; Liu, B.-N.; Zhang, S.-L.; Li, H.; Chen, L.-Q. Acta Chim. Sinica 2015, 73, 808 (in Chinese). (罗飞, 郑杰允, 褚赓, 刘柏男, 张素林, 李泓, 陈立泉, 化学学报, 2015, 73, 808.)
[6] Ye, Y.; Zhu, J.-Y.; Yao, Y.-N.; Wang, Y.-G.; Wu, P.; Tang, Y.-W.; Zhou, Y.-M.; Lu, T.-H. Acta Chim. Sinica 2015, 73, 151 (in Chinese). (叶亚, 朱婧怡, 姚依男, 王雨果, 吴平, 唐亚文, 周益明, 陆天虹, 化学学报, 2015, 73, 151.)
[7] Wen, L.; Liu, C.-M.; Song, R.-S.; Luo, H.-Z.; Shi, Y.; Li, F.; Cheng, H.-M. Acta Chim. Sinica 2014, 72, 333 (in Chinese). (闻雷, 刘成名, 宋仁升, 罗洪泽, 石颖, 李峰, 成会明, 化学学报, 2014, 72, 333.)
[8] Boukamp, B. A.; Lesh, G. C.; Huggins, R. A. J. Electrochem. Soc. 1981, 128, 725.
[9] Obrovac, M. N.; Christensen, L. Electrochem. Solid. ST. 2004, 7, A93.
[10] Chan, C. K.; Ruffo, R.; Hong, S. S.; Huggins, R. A.; Cui, Y. J. Power Sources 2009, 189, 34.
[11] Li, H.; Huang, X. J.; Chen, L. Q.; Zhou, G. W.; Zhang, Z.; Yu, D. P.; Mo, Y. J.; Pei, N. Solid State Ionics 2000, 135, 181.
[12] Yu, Y.; Gu, L.; Zhu, C. B.; Tsukimoto, S.; Aken, P. A.; Maier, J. Adv. Mater. 2010, 22, 2247.
[13] Howe, J. Y.; Burton, D. J.; Qi, Y.; Meyer, H. M.; Nazri, M.; Nazri, G. A.; Palmer, A. C.; Lake, P. D. J. Power Sources 2013, 221, 455.
[14] Sandu, I.; Moreau, P.; Guyomard, D.; Brousse, T.; Roue, L. Solid. State Ionics 2007, 178, 1297.
[15] Ge, M.; Rong, J. P.; Fang, X.; Zhou, C. W. Nano Lett. 2012, 12, 2318.
[16] Abel, P. R.; Lin, Y. M.; Celio, H.; Heller, A.; Mullins, C. B. ACS Nano 2012, 6, 2506.
[17] Chen, D. Y.; Mei, X.; Ji, G.; Lu, M. H.; Xie, J. P.; Lu, J. M.; Lee, J. Y. Angew. Chem. Int. Ed. 2012, 51, 2409.
[18] Li, H.; Huang, X. J.; Chen, L. Q.; Wu, Z. G.; Liang, Y. Electrochem. Solid-State Lett. 1999, 11, 547.
[19] Kim, T. H.; Park, J. S.; Chang, S. K.; Choi, S.; Ryu, J. H.; Song, H. K. Adv. Eng. Mater. 2012, 2, 860.
[20] Wang, M. S.; Song, W. L.; Fan, L. Z. ChemElectroChem. 2015, 2, 1699.
[21] Wang, B.-F.; Yang, J.; Xie, J.-Y.; Wang, K.; Weng, Z.-S.; Yu, X.-G. Acta Chim. Sinica 2003, 61, 1572 (in Chinese). (王保峰, 杨军, 解晶莹, 王可, 文钟晟, 喻献国, 化学学报, 2003, 61, 1572.)
[22] Wang, B.; Li, X. L.; Zhang, X. F.; Luo, B.; Jin, M. H.; Liang, M. H.; Dayeh, S. A.; Picraux, S. T.; Zhi, L. J. ACS Nano 2013, 7, 1437.
[23] Yan, M. Y.; Wang, F. C.; Han, C. H.; Ma, X. Y.; Xu, X.; An, Q. Y.; Xu, L.; Niu, C. J.; Zhao, Y. L.; Tian, X. C.; Hu, P.; Wu, H. G.; Mai, L. Q. J. Am. Chem. Soc. 2013, 135, 18176.
[24] Luo, Z. P.; Xiao, Q. Z.; Lei, G. T.; Li, Z. H.; Tang, C. J. Carbon 2016, 98, 373.
[25] Zhao, K. N.; Zhang, L.; Xia, R.; Dong, Y. F.; Xu, W. W.; Niu, C. J.; He, L.; Yan, M. Y.; Qu, L. B.; Mai, L. Q. Small 2015, 12, 588.
[26] Chou, S. L.; Wang, J. Z.; Choucair, M.; Liu, H. K.; Stride, J. A.; Dou, S. X. Electrochem. Commun. 2010, 12, 303.
[27] Wang, J. Z.; Zhong, C.; Chou, S. L.; Liu, H. K. Electrochem. Commun. 2010, 12, 1467.
[28] Bacon, M.; Bradley, S. J.; Nann, T. Part. Part. Syst. Char. 2014, 31, 415.
[29] Huynh, W. U.; Dittmer, J. J.; Alivisatos, A. P. Science 2002, 295, 2425.
[30] Son, D. I.; Kwon, B. W.; Park, D. H.; Seo, W. S.; Yi, Y.; Angadi, B.; Lee, C. L.; Choi, W. K. Nat. Nanotechnol. 2012, 7, 465.
[31] Lin, J.; Zhang, C. G.; Yan, Z.; Zhu, Y.; Peng, Z. W.; Hauge, R. H.; Natelson, D.; Tour, J. M. Nano. Lett. 2012, 13, 72.
[32] Gao, P.; Ding, K.; Wang, Y.; Ruan, K. Q.; Diao, S. L.; Zhang, Q.; Sun, B. Q.; Jie, J. S. J. Phys. Chem. C 2014, 118, 5164.
[33] Li, R. Y.; Jiang, Y. Y.; Zhou, X. Y.; Li, Z. J.; Gu, Z. G.; Wang, G. L. Electrochim. Acta 2015, 178, 303.
[34] Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I.; Ohta, R.; Okamoto, A. Adv. Mater. 2012, 24, 5333.
[35] Peng, J.; Gao, W.; Gupta, B. K.; Liu, Z.; Aburto, R. R.; Ge, L. H.; Song, L.; Alemany, L. B.; Zhan, X. B.; Gao, G. H.; Vithayathil, S. A.; Kaipparettu, B. A.; Marti, A. A.; Hayashi, T.; Zhu, J. J.; Ajayan, P. M. Nano Lett. 2012, 12, 844.
[36] Jeong, J. H.; Kim, K. H.; Jung, D. W.; Kim, K.; Lee, S. M.; Oh, E. S. J. Power Sources 2015, 300, 182.
/
| 〈 |
|
〉 |