

手性金属-有机骨架材料[Cu(S-mal)(bpy)]n用于高效液相色谱拆分外消旋化合物
收稿日期: 2016-07-16
网络出版日期: 2016-09-18
基金资助
项目受国家自然科学基金(Nos.21275126,21365024)和云南省基础研究项目(No.2013FB035)资助.
Chiral Metal-Organic Framework [Cu(S-mal)(bpy)]n Used for Separation of Racemates in High Performance Liquid Chromatography
Received date: 2016-07-16
Online published: 2016-09-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21275126, 21365024) and the Yunnan Province's Basic Research Project (No. 2013FB035).
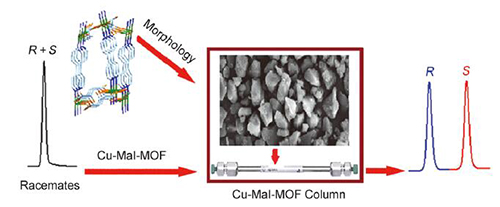
手性金属-有机骨架材料作为一种新型的多孔材料,在手性分离领域备受关注.以S-苹果酸和4,4'-联吡啶为配体与醋酸铜反应,通过溶剂热法合成了一种具有三维手性网状结构的手性金属-有机骨架材料[Cu(S-mal)(bpy)]n.将其用作固定相制成高效液相色谱手性柱,采用不同比例的正己烷/异丙醇作为流动相对一系列外消旋化合物进行手性拆分.结果显示,该手性柱对醇类、酮类、黄酮、酚类、胺类等17种外消旋化合物表现了较好的手性拆分能力.将该手性柱与之前我们报道的3种手性MOFs柱相比较,其表现出了更好的手性选择性,具有较好的手性拆分互补作用.最后还对该手性柱的重现性和稳定性做了评价,结果表明该手性柱表现出较好的重现性和稳定性.
胡聪 , 李丽 , 杨娜 , 张紫恒 , 谢生明 , 袁黎明 . 手性金属-有机骨架材料[Cu(S-mal)(bpy)]n用于高效液相色谱拆分外消旋化合物[J]. 化学学报, 2016 , 74(10) : 819 -824 . DOI: 10.6023/A16070349
Chiral metal-organic framework materials, as a new type of porous materials, have attracted much attention in the field of chiral separation. In this paper, a homochiral MOF [Cu(S-mal)(bpy)]n with 3D chiral networks was synthesized by the reaction of ligands (S-malic acid and 4,4'-bipyridine) with copper acetate via a solvothermal method. A packed chiral column for high performance liquid chromatography was fabricated using [Cu(S-mal)(bpy)]n as stationary phase. Before the packing, the MOF crystals was crushed in ethanol applying soft pressure and then the MOF with suitable particle size (5~10 m) was obtained via solvent suspension. A 4.2 g mass of prepared MOF was suspended in a mixture of hexane and isopropanol. In order to control the packing quality, the suspension of MOF was packed into a stainless steel empty column (25 cm long×4.6 mm i.d.) under 40 MPa using hexane/isopropanol (9:1, V/V) as the slurry solvent according to a conventional high pressure slurry packing procedure. To investigate the chiral recognition ability of this stationary phase, a series of racemic compounds were separated on the chiral MOF column using different ratio of n-hexane/isopropanol as mobile phase. The results showed that the chiral column exhibited good resolving ability towards 17 racemates, including alcohols, ketones, flavonoids, phenols and amines. For instance, the resolution value of 1-(1-naphthyl)ethanol could reach 4.5. Compared with three kinds of homochiral MOFs columns previously reported by our group, this column showed better chiral recognition ability and higher resolution toward racemates, and has a good complementary for chiral separation. The [Cu(S-mal)(bpy)]n possesses cavities with average dimensions (5 Å×5 Å×6 Å), which were interconnected by narrow windows with diameter ≤3 Å. Therefore, the chiral recognition mostly depends on the surface of the MOF crystal in which the steric fit between the chiral networks and conformation of the solute molecule is the main interactive force. Besides, many other interactions such as the hydro-gen-bondings, dispersion forces, dipole-dipole interaction, and π-π interactions which come from the solutes, chiral stationary phase and the mobile phase may also play some role. The reproducibility and stability of the chiral column were evaluated. The results showed that the chiral column showed good reproducibility and stability for enantioseparation.

[1] Wei, W. Y.; Fang, J.; Kong, H. N.; Han, J. Y.; Chang, H. Y. Prog. Chem. 2005, 17, 1110(in Chinese). (魏文英, 方键, 孔海宁, 韩金玉, 常贺英, 化学进展, 2005, 17, 1110.)
[2] Mu, C. Z.; Xu, F.; Lei, W. Prog. Chem. 2007, 19, 1345(in Chinese). (穆翠枝, 徐峰, 雷威, 化学进展, 2007, 19, 1345.)
[3] Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. J. Mater. Chem. 2006, 16, 626.
[4] Qi, X. Y.; Li, X. J.; Bai, Y.; Liu, H. W. Chin. J. Chromatogr. 2016, 34, 10(in Chinese). (祁晓月, 李先江, 白玉, 刘虎威, 色谱, 2016, 34, 10.)
[5] Xie, S. M.; Yuan, L. M. Prog. Chem. 2013, 25, 1763(in Chinese). (谢生明, 袁黎明, 化学进展, 2013, 25, 1763.)
[6] Li, X. J.; He, C. F.; Huang, B.; Lin, Z. Y. Chem. Ind. Eng. Prog. 2016, 35, 586(in Chinese). (李小娟, 何长发, 黄斌, 林振宇, 刘以凡, 林春香, 化工进展, 2016, 35, 586.)
[7] Pham, M. H.; Vuong, G. T.; Fontaine, F. G. Cryst. Growth Des. 2011, 12, 1008.
[8] Lee, J. Y.; Pan, L.; Huang, X. Y. Adv. Funct. Mater. 2011, 21, 993.
[9] Xiang, Z. H.; Hu, Z.; Cao, D. P. Angew. Chem., Int. Ed. 201l, 50, 491.
[10] Bao, Z.; Yu, L.; Ren, Q. J. Coll. Inter. Sci. 2011, 353, 549.
[11] Kim, H.; Park, J.; Jung, Y. Phys. Chem. Chem. Phys. 2013, 15, 19644.
[12] Wang, B.; Lv, X. L.; Feng, D. W.; Xie, L. H.; Zhang, J.; Li, M.; Xie, Y. B.; Li, J. R.; Zhou, H. C. J. Am. Chem. Soc. 2016, 138, 6204.
[13] Jia, J. T.; Wang, L.; Zhao, Q.; Sun, F. X.; Zhu, G. S. Acta Chim. Sinica 2013, 71, 1492(in Chinese). (贾江涛, 王蕾, 赵晴, 孙福兴, 朱广山, 化学学报, 2013, 71, 1492.)
[14] Xu, J.; Shimakoshi, H.; Hisaeda, Y. J. Organomet. Chem. 2015, 782, 89.
[15] Karimi, Z.; Morsali, A. J. Mater Chem. A 2013, 1, 3047.
[16] Guo, R. M.; Bai, J. Q.; Zhang, H.; Xie, Y. B.; Li, J. R. Prog. Chem. 2016, 28, 232(in Chinese). (郭瑞梅, 白金泉, 张恒, 谢亚勃, 李建荣, 化学进展, 2016, 28, 232.)
[17] Huang, G.; Chen, Y. Z.; Jiang, H. L. Acta Chim. Sinica 2016, 74, 113(in Chinese). (黄刚, 陈玉贞, 江海龙, 化学学报, 2016, 74, 113.)
[18] Qian, J. J.; Qiu, L. G.; Wang, Y. M. Dalton Trans. 2014, 43, 3978.
[19] Nickerl, G.; Senkovska, I.; Kaskel, S. Chem. Commun. 2015, 51, 2280.
[20] Ezuhara, T.; Endo, K.; Aoyama, Y. J. Am. Chem. Soc. 1999, 121, 3279.
[21] Dai, R. J.; Tong, B.; Tang, L.; Deng, Y. L.; Fu, R. N. Acta Chim. Sinica 2006, 64, 1248(in Chinese). (戴荣继, 佟斌, 唐力, 邓玉林, 傅若农, 化学学报, 2006, 64, 1248.)
[22] Gu, Z. Y.; Jiang, D. Q.; Wang, H. F.; Cui, X. Y.; Yan, X. P. J. Phys. Chem. C 2010, 114, 311.
[23] Gu, Z. Y.; Yang, C. X.; Chang, N.; Yan, X. P. Acc. Chem. Res. 2012, 45, 734.
[24] Li, J. R.; Sculley, J.; Zhou, H. C. Chem. Rev. 2012, 112, 869.
[25] Gu, Z. Y.; Yan, X. P. Angew. Chem., Int. Ed. 2010, 49, 1477.
[26] Zhao, W. W.; Zhang, C. Y.; Yan, Z. G.; Bai, L. P.; Wang, X. Y.; Huang, H. L.; Zhou, Y. Y.; Xie, Y. B.; Li, F. S.; Li, J. R. J. Chromatogr. A 2014, 1370, 121.
[27] Zhu, Z. J.; Wang, Q. Q.; Kang, J. W. Acta Chim. Sinica 2008, 66, 1845(in Chinese). (朱智甲, 王倩倩, 康经武, 化学学报, 2008, 66, 1845.)
[28] Ezuhara, T.; Endo, K.; Aoyama, Y. J. Am. Chem. Soc. 1999, 121, 3279.
[29] Nuzhdin, A. L.; Dybtsev, D. N.; Bryliakov, K. P. J. Am. Chem. Soc. 2007, 129, 12958.
[30] Padmanaban, M.; Müller, P.; Lieder, C.; Gedrich, K.; Grunker, R.; Bon, V.; Senkovska, I.; Baumgartner, S.; Opelt, S.; Paasch, S.; Brunner, E.; Glorius, F.; Klemm, E.; Kaskel, S. Chem. Commun. 2011, 47, 12089.
[31] Tanaka, K.; Muraoka, T.; Hirayama, D.; Ohnish, A. Chem. Commun. 2012, 48, 8577.
[32] Zhou, L. L.; Sun, W. Z.; Wang, J. Y.; Yuan, L. M. Acta Chim. Sinica 2008, 66, 2309(in Chinese). (周玲玲, 孙文卓, 王剑瑜, 袁黎明, 化学学报, 2008, 66, 2309.)
[33] Zhang, M.; Pu, Z. J.; Chen, X. L.; Gong, X. L.; Zhu, A. X.; Yuan, L. M. Chem. Commun. 2013, 49, 5201.
[34] Zhang, M.; Zhang, J. H.; Zhang, Y.; Wang, B. J.; Xie, S. M.; Yuan, L. M. J. Chromatogr. A 2014, 1325, 163.
[35] Kong, J.; Zhang, M.; Duan, A. H.; zhang, J. H.; Yang, R.; Yuan, L. M. J. Sep. Sci. 2015, 38, 556.
[36] Nong, R. Y.; Kong, J.; Zhang, J. H.; Chen, L.; Tang, B.; Xie, S. M.; Yuan, L. M. Chem. J. Chin. Univ. 2016, 37, 19(in Chinese). (农蕊瑜, 孔娇, 章俊辉, 陈玲, 汤波, 谢生明, 袁黎明, 高等学校化学学报, 2016, 37, 19.)
[37] Ma, S.; Shen, S.; Lee, H.; Eriksson, M.; Zeng, X.; Xu, J.; Fandrick, K.; Yee, N.; Senanayake, C.; Grinberg, N. J. Chromatogr. A 2009, 1216, 3784.
[38] Chankvetadze, B. J. Chromatogr. A 2012, 1269, 26.
[39] Zavakhina, M. S.; Samsonenko, D. G.; Virovets, A. V.; Dybtsev, D. N.; Fedin, V. P. J. Solid State Chem. 2014, 210, 125.
[40] Xie, S. M.; Zhang, X. H.; Zhang, Z. J.; Yuan, L. M. Anal. Lett. 2013, 46, 753.
/
| 〈 |
|
〉 |