

用原位电镜技术研究银纳米线与硫溶液的液相反应机理
收稿日期: 2016-10-24
网络出版日期: 2016-12-05
基金资助
项目受国家自然科学基金(No.21433013)和中国科学院国际合作局对外合作重点项目(No.121E32KYSB20150004)资助.
In-situ TEM Study of the Liquid-Phase Reaction of Ag Nanowires with a Sulfur Solution
Received date: 2016-10-24
Online published: 2016-12-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21433013) and the CAS-DOE Joint Research Program (No. 121E32KYSB20150004).
荣根兰 , 张心怡 , 胥燕 , 张跃钢 . 用原位电镜技术研究银纳米线与硫溶液的液相反应机理[J]. 化学学报, 2016 , 74(12) : 980 -983 . DOI: 10.6023/A16100562
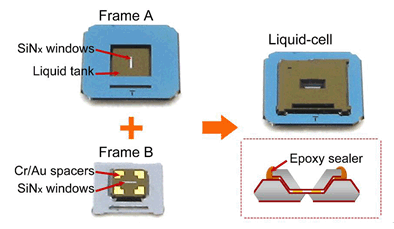
Using transmission electron microscopy (TEM) to directly observe a dynamic chemical reaction process in liquid-phase environment is a big challenge because it is difficult to keep liquid reactants under vacuum.In this work,we reported a liquid-based cell that enables in-situ observation of a solution reaction process under TEM.The novel liquid cell design not only realizes the self-alignment of top/bottom windows,but also achieves an adjustable liquid layer thickness down to nanometer scale.The cell consists of top and bottom frames,both of which are fabricated from silicon wafers using conventional micro-fabrication techniques.The transparent observation windows are made of silicon nitride (SiNx) membranes.In a typical assembling process,an ethanol solution containing silver nanowires and an ethanol solution containing saturated sulfur were sequentially dropped into the liquid tank of the bottom-frame of a liquid cell by using 1 mL syringe.Then,the liquid tank was covered by the top-frame with a window direction of 90 degrees,and an epoxy was used to seal the edge between the top-frame and bottom-frame.Using this assembled liquid cell,we performed in-situ TEM observation of the chemical reaction between Ag nanowires and sulfur in ethanol solution.Additional ex-situ X-ray diffraction (XRD) and Raman spectroscopy were also performed to study the reaction intermediates and final products.Instead of a simple reaction process in which sulfur diffuses into Ag to form the final product of Ag2S,we found that the real reaction process involves the formation of a soluble intermediate phase (Ag2S4),which led to a partial dissolution of Ag nanowires in ethanol solution during reaction.These results well demonstrate that the in-situ TEM technique is a powerful tool to reveal the "real" chemical reaction mechanism.The experimental technique developed here could also be used to study a broad range of dynamic phenomena in liquid environment.
[1] Xu, T.; Sun, L. Small 2015, 11, 3247.
[2] Zhu, G.; Jiang, Y.; Huang, W.; Zhang, H.; Lin, F.; Jin, C. Chem. Commun. 2013, 49, 10944.
[3] Liao, H. G.; Cui, L.; Whitelam, S.; Zheng, H. Science 2012, 336, 1011.
[4] Zeng, Z.; Liang, W. I.; Liao, H. G.; Xin, H. L.; Chu, Y. H.; Zheng, H. Nano Lett. 2014, 14, 1745.
[5] Jungjohann, K. L.; Bliznakov, S.; Sutter, P. W.; Stach, E. A.; Sutter, E. A. Nano Lett. 2013, 13, 2964.
[6] de Jonge N.; Peckys, D. B.; Kremers, G. J.; Piston, D. W. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 2159.
[7] Meng, Z.-D.; Ghosh, T.; Zhu, L.; Choi, J.-G.; Park, C.-Y.; Oh, W.-C. J. Mater. Chem. 2012, 22, 16127.
[8] Yan, S.; Shen, K.; Xu, X.; Shi, Y.; Wu, J.; Xiao, Z. Synth. Met. 2011, 161, 1646.
[9] Bourret, G. R.; Lennox, R. B. Nanoscale 2011, 3, 1838.
[10] Liao, Z. M.; Hou, C.; Zhao, Q.; Wang, D. S.; Li, Y. D.; Yu, D. P. Small 2009, 5, 2377.
[11] Wang, D.; Hao, C.; Zheng, W.; Peng, Q.; Wang, T.; Liao, Z.; Yu, D.; Li, Y. Adv. Mater. 2008, 20, 2628.
[12] Gao, F.; Lu, Q.; Zhao, D. Nano Lett. 2003, 3, 85.
[13] Lee, U.; Ham, S.; Han, C.; Jeon, Y. J.; Myung, N.; Rajeshwar, K. Mater. Chem. Phys. 2010, 121, 549.
[14] Choi, O.; Cleuenger, T. E.; Deng, B. L.; Surampalli, R. Y.; Ross, L.; Hu, Z. Q. Water Res. 2009, 43, 1879.
[15] Huang, T. W.; Liu, S. Y.; Chuang, Y. J.; Hsieh, H. Y.; Tsai, C. Y.; Huang, Y. T.; Mirsaidov, U.; Matsudaira, P.; Tseng, F. G.; Chang, C. S.; Chen, F. R. Lab Chip 2012, 12, 340.
[16] Yuk, J. M.; Seo, H. K.; Choi, J. W.; Lee, J. Y. ACS Nano 2014, 8, 7478.
[17] Seino, S.; Imoto, Y.; Kitagawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Yamamoto, T. A. J. Nucl. Sci. Technol. 2016, 53, 1021.
[18] Song, M. K.; Zhang, Y.; Cairns, E. J. Nano Lett. 2013, 13, 5891.
[19] Nie, Y. H.; Zhang, B.; Zhou, X. W.; Zhao, L. G. Anal. Lab. 2016, 35, 813. (聂钰洪, 张蓓, 周晓微, 赵凌国, 分析试验室, 2016, 35, 813.)
[20] Klein, K. L.; Anderson, I. M.; De Jonge, N. J. Microsc. 2011, 242, 117.
/
| 〈 |
|
〉 |