

过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展
收稿日期: 2019-05-14
网络出版日期: 2019-07-16
基金资助
项目受国家自然科学基金重点项目资助(No. 21732003)
Advances on Transition Metals and Photoredox Cooperatively Catalyzed Allylic Substitutions
Received date: 2019-05-14
Online published: 2019-07-16
Supported by
Project supported by the National Natural Science Foundation of China(No. 21732003)
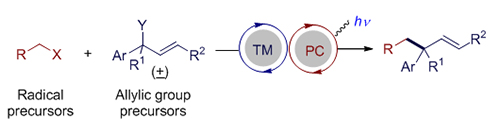
过渡金属催化的烯丙基取代反应是一类重要且实用的有机化学反应, 可以立体选择性地高效构建碳-碳键和碳-杂键. 可见光氧化还原催化可以利用绿色清洁的可见光能源在较为温和的条件下产生自由基或者自由基离子等高反应活性的反应中间体, 被广泛地应用于有机合成中, 逐渐发展成为一种重要的合成工具. 鉴于烯丙基取代反应的重要性, 过渡金属与光氧化还原协同催化的烯丙基取代反应逐渐引起化学家的兴趣. 该协同催化的策略可以实现单一过渡金属催化难以实现的烯丙基取代反应, 反应的区域选择性和立体选择性也体现出不同的特点, 有望发展成为单一金属催化的烯丙基取代反应的重要补充. 本文综述了近年来不同过渡金属与可见光氧化还原协同催化的烯丙基取代反应的研究进展.
张洪浩 , 俞寿云 . 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019 , 77(9) : 832 -840 . DOI: 10.6023/A19050177
Allylic substitutions catalyzed by transition metals are important and practical reactions, which can construct carbon-carbon bonds and carbon-heteroatom bonds efficiently and stereoselectively. Various transition metal catalysts, such as Pd, Ir, Cu, Ni, Rh and Ru, have been widely used in this reaction. To date, various “soft”, or stabilized nucleophiles (pKa<25), including malonates, acetoacetates and enolates, have been used in allylic substitutions. Conversely, the high reactivity of “hard”, or non-stabilized alkyl nucleophiles (pKa>25) has limited their utility in catalytic processes and their compatibility with functional groups. Visible light photoredox catalysis has been widely used in organic synthesis because it can generate high reactive intermediates, such as free radicals and radical ions, under mild conditions using green and clean energy, and has gradually developed into an important synthetic tool. Furthermore, merging photoredox catalysis with transition metal catalysts has become a popular strategy for expanding the synthetic utility of visible-light photocatalysis, and has led to the discovery of novel reaction modes. Due to the high activity of the intermediates in photoredox catalysis, the selectivity of these reactions, especially stereoselectivity, is still a challenge. In view of the importance of allyl substitutions, the allyl substitution co-catalyzed by transition metals and photoredox has attracted the interest of chemists. The synergistic strategy can realize allylic substitutions which are difficult to be achieved by single transition metal catalysis. The regioselectivity and stereoselectivity of these reactions also show different characteristics. It is expected to become an important complement to allylic substitution catalyzed by single metal. In this review, recent advances on allylic substitution co-catalyzed by different transition metals and photoredox are summarized. Meanwhile, the mechanism of representative transformations will be briefly introduced and the prospective in this area will be given.

| [1] | (a) Tsuji, J.; Takahashi, H.; Morikawa, M . Tetrahedron Lett. 1965, 6, 4387. |
| [1] | (b) Tsuji, J . Acc. Chem. Res. 1969, 2, 144. |
| [2] | Trost, B. M.; Strege, P. E. J. Am. Chem. Soc. 1977, 99, 1649. |
| [3] | (a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395. |
| [3] | (b) Trost B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921. |
| [3] | (c) Trost, B. M.; Machacek, M. R.; Aponick, A. Acc. Chem. Res. 2006, 39, 747. |
| [3] | (d) Trost, B. M. Org. Process Res. Dev. 2012, 16, 185. 185. |
| [4] | (a) Wang, Y-N.; Lu, L.-Q.; Xiao, W.-J. Chem. Asian J. 2018, 13, 2174. |
| [4] | (b) Tang, H.; Huo, X.; Meng, Q.; Zhang, W. Acta Chim. Sinica 2016, 74, 219 (in Chinese). |
| [4] | (汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.) |
| [5] | (a) Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461. |
| [5] | (b) Cheng, Q.; Tu, H. F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. . Chem Rev. 2019, 119, 1855. |
| [5] | (c) Deng, Y.; Yang, W.; Yang, X.; Yang, D.Chin. J. Org. Chem. 2017, 37, 3039 (in Chinese). |
| [5] | (邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.) |
| [6] | (a) Alexakis, A.; B?ckvall, J. E.; Krause, N.; Pàmies, O.; Diéguez, M. . Chem Rev. 2008, 108, 2796. |
| [6] | (b) Yorimitsu, H.; Oshima, K. Angew. Chem., Int. Ed. 2005, 44, 4435. |
| [7] | Zhang, H.; Gu, Q.; You, S.-L . Chin. J. Org. Chem. 2019, 39, 15 (in Chinese). |
| [7] | ( 张慧君, 顾庆, 游书力 , 有机化学, 2019, 39, 15.) |
| [8] | (a) Turnbull, B. W. H.; Evans, P. A. J. Org. Chem. 2018, 83, 11463. |
| [8] | (b) Thoke, M. B.; Kang, Q. Synthesis 2019, DOI: 10. 1055/s-0037-1611784. |
| [9] | Bruneau, C.; Renaud, J.-L.; Demerseman, B. Chem. Eur. J. 2006, 12, 5178. |
| [10] | (a) Trost, B. M.; Verhoeven, T. R. J. Org. Chem. 1976, 41, 3215. |
| [10] | (b) Matsushita, H.; Negishi, E. J. Chem. Soc. Chem. Commun. 1982, 160. |
| [10] | (c) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258. |
| [10] | (d) Weaver, J. D.; Recio, III, A.; Grenning, A. J.; Tunge, J. A. Chem. Rev. 2011, 111, 1846. |
| [10] | (e) Yu, Y.-N.; X, M.-H. Acta Chim. Sinica 2017, 75, 655 (in Chinese). (于月娜, 徐明华, 化学学报, 2017, 75, 655.) |
| [10] | (f) Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synjournal, Ed.: Kazmaier, U., Springer, Heidelberg, 2012. |
| [11] | For reviews on this topic, see: (a) Harutyunyan, S. R.; den Hartog, T.; Geurts, K.; Minnaard, A. J.; Feringa, B. L. Chem. Rev. 2008, 108, 2824. |
| [11] | (b) Alexakis, A.; Backvall, J. E.; Krause, N.; Pa?mies, O.; Die?guez, M . Chem. Rev. 2008, 108, 2796. |
| [11] | (c) Teichert, J. F.; Ferin-ga, B. L. Angew. Chem., Int. Ed. 2010, 49, 2486. |
| [11] | (d) Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. Chem. Rev. 2015, 115, 9587. |
| [11] | (e) Hartwig, J. F.; Pouy, M. J. Top. Organomet. Chem. 2011, 34, 169. |
| [11] | (f) Scha?fer, P.; Sidera, M.; Palacin, T.; Fletcher, S. P. Chem. Commun. 2017, 53, 12499. |
| [12] | (a) Zheng, W.-H.; Zheng, B.-H.; Zhang, Y.; Hou, X.-L. J. Am. Chem. Soc. 2007, 129, 7718. |
| [12] | (b) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2008, 130, 14092. |
| [12] | (c) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2009, 131, 12056. |
| [12] | (d) Zhang, P.; Brozek, L. A.; Morken, J. P. J. Am. Chem. Soc. 2010, 132, 10686. |
| [12] | (e) Chen, J.-P.; Ding, C.-H.; Liu, W.; Hou, X.-L.; Dai, L.-X. J. Am. Chem. Soc. 2010, 132, 15493. |
| [12] | (f) Zhang, P.; Le, H.; Kyne, R. E.; Morken, J. P. J. Am. Chem. Soc. 2011, 133, 9716. |
| [12] | (g) Trost, B. M.; Thaisrivongs, D. A.; Hartwig, J. J. Am. Chem. Soc. 2011, 133, 12439. |
| [12] | (h) Chen, J.-P.; Peng, Q.; Lei, B.-L.; Hou, X.-L.; Wu, Y.-D. J. Am. Chem. Soc. 2011, 133, 14180. |
| [12] | (i) Brozek, L. A.; Ardolino, M. J.; Morken, J. P. J. Am. Chem. Soc. 2011, 133, 16778. |
| [12] | (j) Ardolino, M. J.; Morken, J. P. J. Am. Chem. Soc. 2014, 136, 7092. |
| [12] | (k) Niyomchon, S.; Audisio, D.; Luparia, M.; Maulide, N. Org. Lett. 2013, 15, 2318. |
| [12] | (l) Misale, A.; Niyomchon, S.; Luparia, M.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 7068. |
| [12] | (m) Mao, J.; Zhang, J.; Jiang, H.; Bellomo, A.; Zhang, M.; Gao, Z.; Dreher, S. D.; Walsh, P. J. Angew. Chem., Int. Ed. 2016, 55, 2526. |
| [12] | (n) Murakami, R.; Sano, K.; Iwai, T.; Taniguchi, T.; Monde, K.; Sawamura, M. Angew. Chem., Int. Ed. 2018, 57, 9465. |
| [13] | (a) Butt, N.; Yang, G.; Zhang, W. Chem . Rec. 2016, 16, 2687. |
| [13] | (b) Afewerki, S.; Córdova, A. Chem . Rev. 2016, 116, 13512. |
| [13] | (c) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838 (in Chinese). |
| [13] | (张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76, 838.) |
| [13] | (d) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929. |
| [13] | (e) Fu, J.; Huo, X.; Li, B.; Zhang, W. Org. Biomol. Chem. 2017, 15, 9747. |
| [14] | For reviews on photoredox catalysis, see: (a) Narayanam, J. M. R.; Ste-phenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102. |
| [14] | (b) Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828. |
| [14] | (c) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [14] | (d) Dai, X.-J.; Xu, X.-L.; Li, X.-N. Chin. J. Org. Chem. 2013, 33, 2046 (in Chinese). |
| [14] | (戴小军, 许孝良, 李小年, 有机化学, 2013, 33, 2046.) |
| [14] | (e) Schultz, D. M.; Yoon, T. P. Science 2014, 343, 1239176. |
| [14] | (f) Brim-ioulle, R.; Lenhart, D.; Maturi, M. M.; Bach, T. Angew. Chem., Int. Ed. 2015, 54, 3872. |
| [14] | (g) Tan, F.; Xiao, W. Acta Chim. Sinica 2015, 73, 85 (in Chinese). |
| [14] | (谭芬, 肖文精, 化学学报, 2015, 73, 85.) |
| [14] | (h) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075. |
| [14] | (i) Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850. |
| [15] | For selected examples on palladium metallaphotoredox catalysis, see: (a) Kalyani, D.; McMurtrey, K. B.; Neufeldt, S. R.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18566. |
| [15] | (b) Neufeldt, S. R.; Sanford, M. S. Adv. Synth. Catal. 2012, 354, 3517. |
| [15] | (c) Zoller, J.; Fabry, D. C.; Ronge, M. A.; Rueping, M. . Angew. Chem., Int. Ed. 2014, 53, 13264. |
| [15] | (d) Mori, K.; Kawashima, M.; Yamashita, H . Chem. Commun. 2014, 50, 14501. |
| [15] | (e) Choi, S.; Chatterjee, T.; Choi, W. J.; You, Y.; Cho, E. J . ACS Catal. 2015, 5, 4796. |
| [15] | (f) Zhou, C.; Li, P.; Zhu, X.; Wang, L . Org. Lett. 2015, 17, 6198. |
| [15] | (g) Cheng, W.-M.; Shang, R.; Yu, H.-Z.; Fu, Y . Chem. Eur. J. 2015, 21, 13191. |
| [15] | (h) Liu, K.; Zou, M.; Lei, A . J. Org. Chem. 2016, 81, 7088. |
| [15] | (i) K?rk?s, M. D.; Bosque, I.; Matsuura, B. S.; Stephenson, C. R. J . Org. Lett. 2016, 18, 5166. |
| [15] | (j) Shimomaki, K.; Murata, K.; Martin, R.; Iwasawa, N . J. Am. Chem. Soc. 2017, 139, 9467. |
| [15] | (k) Kato, S.; Saga, Y.; Kojima, M.; Fuse, H.; Matsunaga, S.; Fukatsu, A.; Kondo, M.; Masaoka, S.; Kanai, M . J. Am. Chem. Soc. 2017, 139, 2204. |
| [16] | For selected examples on nickel metallaphotoredox catalysis, see: (a) Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; MacMillan, D. W. C. Science 2014, 345, 437. |
| [16] | (b) Tellis, J. C.; Primer, D. N.; Molander, G. A . Science 2014, 345, 433. |
| [16] | (c) Corcé, V.; Chamoreau, L.-M.; Derat, E.; Goddard, J.-P.; Ollivier, C.; Fensterbank, L . Angew. Chem., Int. Ed. 2015, 54, 11414. |
| [16] | (d) Nakajima, K.; Nojima, S.; Nishibayashi, Y . Angew. Chem., Int. Ed. 2016, 55, 14106. |
| [16] | (e) Shaw, M. H.; Shurtleff, V. W.; Terrett, J. A.; Cuthbertson, J. D.; MacMillan, D. W. C . Science. 2016, 352, 1304. |
| [16] | (f) Heitz, D. R.; Tellis, J. C.; Molander, G. A . J. Am. Chem. Soc. 2016, 138, 12715. |
| [17] | For selected examples on copper metallaphotoredox catalysis, see: (a) Ye, Y.; Sanford, M. S. J. Am. Chem. Soc. 2012, 134, 9034. |
| [17] | (b) Yoo, W.-J.; Tsukamoto, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2015, 54, 6587. |
| [18] | For selected examples on gold metallaphotoredox catalysis, see: (a) Sahoo, B.; Hopkinson, M. N.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 5505. |
| [18] | (b) Shu, X.-Z.; Zhang, M.; He, Y.; Frei, H.; Toste, F. D. J. Am. Chem. Soc. 2014, 136, 5844. |
| [19] | (a) Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035. |
| [19] | (b) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A . Acc. Chem. Res. 2016, 49, 1429.. |
| [19] | (c) Twilton, J.; Le, C.; Zhang, P.; Shaw, M. H.; Evans, R. W.; MacMillan, D. W. C . Nature Rev. 2017, 1, 0052. |
| [19] | (d) Wang, C.-S.; Dixneuf, P. H.; Soule?, J.-F . Chem. Rev. 2018, 118, 7532. |
| [19] | (e) Zhou, W.-J.; Zhang, Y.-H.; Gui, Y.-Y.; Sun, L.; Yu, D.-G . Synthesis. 2018, 50, 3359. |
| [19] | (f) Chuentragool, P.; Kurandina, D.; Gevorgyan, V . Angew. Chem., Int. Ed. 2019, DOI: 10. 1002/anie. 201813523. |
| [20] | Lang, S. B.; O’Nele, K. M.; Tunge, J. A. J. Am. Chem. Soc. 2014, 136, 13606. |
| [21] | Lang, S. B.; O’Nele, K. M.; Tunge, J. A. Chem. Eur. J. 2015, 21, 18589. |
| [22] | Xuan, J.; Zeng, T.-T.; Feng,, Z,-J.; Deng, Q.-H.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J.; Alper, H. Angew. Chem., Int. Ed. 2015, 54, 1625. |
| [23] | Jennifer, K. Matsui, J. K.; Gutiérrez-Bonet, A.; Rotella, M.; Alam, R.; Gutierrez, O.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57, 15847. |
| [24] | Thullen, S. M.; Rovis, T. J. Am. Chem. Soc. 2017, 139, 15504. |
| [25] | Zheng, J.; Breit, B. Angew. Chem., Int. Ed. 2019, 58, 3392. |
| [26] | Schwarz, J. L.; Scha?fers, F.; Tlahuext-Aca, A.; Lu?ckemeier, L.; Glorius, F. J. Am. Chem. Soc. 2018, 140, 12705. |
| [27] | For selected examples on asymmetric nickel metallaphotoredox catalysis, see:(a) Zuo, Z.; Cong, H.; Li, W.; Choi, J.; Fu, G. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 138, 1832. |
| [27] | (b) Stache, E. E.; Rovis, T.; Doyle, A. G . Angew. Chem., Int. Ed. 2017, 56, 3679. For selected examples on asymmetric copper metallaphotoredox catalysis, see: |
| [27] | (c) Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc. 2017, 139, 15632. |
| [27] | (d) Sha, W.; Deng, L.; Ni, S.; Mei, H.; Han, J.; Pan, Y . ACS Catal. 2018, 8, 7489. |
| [28] | Zhang, H.-H.; Zhao, J.-J.; Yu, S. J. Am. Chem. Soc. 2018, 140, 16914. |
| [29] | Mitsunuma, H.; Tanabe, S.; Fuse, H.; Ohkubo, K.; Kanai, M . Chem. Sci. 2019, 10, 3459. |
/
| 〈 |
|
〉 |