

铜催化苯甲酰亚胺高烯丙酯的分子内胺化全氟烷基化反应
收稿日期: 2019-06-18
网络出版日期: 2019-08-13
基金资助
项目受国家自然科学基金(Nos.21672105);项目受国家自然科学基金(21702109);项目受国家自然科学基金(21890722);天津市自然科学基金(Nos.17JCYBJC19700);天津市自然科学基金(18JCZDJC32800);南开大学中央高校基本科研业务费专项资金资助(No.63161122)
Copper-catalyzed Intramolecular Aminoperfluoroalkylation Reaction of O-Homoallyl Benzimidates
Received date: 2019-06-18
Online published: 2019-08-13
Supported by
Project supported by the National Natural Science Foundation of China(Nos.21672105);Project supported by the National Natural Science Foundation of China(21702109);Project supported by the National Natural Science Foundation of China(21890722);Natural Science Foundation of Tianjin(Nos.17JCYBJC19700);Natural Science Foundation of Tianjin(18JCZDJC32800);the Fundamental Research Funds for the Central Universities (Nankai University)(No.63161122)
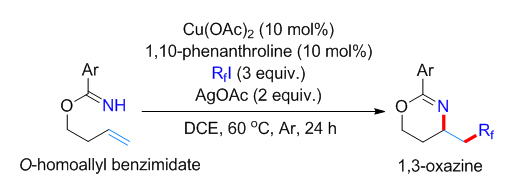
报道了铜催化苯甲酰亚胺高烯丙酯底物的分子内胺化全氟烷基化反应. 该反应以全氟碘代烷为全氟烷基化试剂, 醋酸铜为催化剂, 邻菲啰啉为配体, 在醋酸银存在下以中等的收率实现苯甲酰亚胺高烯丙酯底物末端双键的胺化全氟烷基化, 最终生成1,3-噁嗪类分子. 多种官能团取代的苯甲酰亚胺高烯丙酯和具有不同碳链长度的全氟碘代烷烃都能适用于该反应, 为多氟烷基取代的1,3-噁嗪类化合物的合成提供了一种简洁的方法. 多氟烷基取代的1,3-噁嗪类化合物还可在温和条件下高效转化为γ氨基醇衍生物. 初步的机理研究证明该反应经历了全氟烷基自由基对碳碳双键的亲电加成, 之后苯甲酰亚胺基团作为分子内亲核性胺源经历分子内亲核取代途径生成1,3-噁嗪骨架.
张衡 , 牟学清 , 陈弓 , 何刚 . 铜催化苯甲酰亚胺高烯丙酯的分子内胺化全氟烷基化反应[J]. 化学学报, 2019 , 77(9) : 884 -888 . DOI: 10.6023/A19060220
Azaheterocycles have been broadly applied in the development of therapeutic agents, agrochemicals and functional material molecules. Azaheterocycles equipped with perfluoroalkyl group usually manifest superior physical and biological properties than their parent molecules, such as showing improved metabolic stability and high lipophilicity. The synthesis of perfluoroalkyl modified azaheterocycles has attracted considerable research interest in recent years. The strategy of intramolecular aminoperfluoroalkylation of alkenes, which functionalize C=C bond with an external perfluoroalkyl group and an internal amine nucleophile in one pot, provides a streamlined synthesis of perfluoroalkyl substituted azaheterocycles. This strategy has been applied by Liu, Sodeoka and other research groups in the synthesis of perfluoroalkyl substituted aziridines, pyrrolidines, lactams and pyrazolines featuring the use of pendent amine, amide, hydrazone or urea group as internal amine source. We have previously developed a copper(I)-catalyzed intramolecular aminotrifluoromethylation reaction of O-homoallyl benzimidates with Togni reagent I for the synthesis of trifluoromethyl containing chiral 1,3-oxazines using a chiral BOX ligand. However, this method is limited to aminotrifluoromethylation reaction as other perfluoroalkyl substituted hypervalent iodine reagents are not easily accessible. Herein, we report our recent research results on the synthesis of perfluoroalkyl substituted 1,3-oxazines using commercial available perfluoroalkyl iodides as perfluoroalkyl source. This intramolecular aminoperfluoroalkylation reaction proceeds selectively in the presence of Cu(OAc)2 catalyst, 1,10-phenanthroline ligand and AgOAc additive. A broad range of O-homoallyl benzimidates and perfluoroalkyl iodides are compatible with the reaction conditions, affording perfluoroalkyl substituted 1,3-oxazines in moderate to good yields. The 1,3-oxazine product can be prepared in gram scale and readily hydrolyzed under mild conditions to give perfluoroalkyl substituted 1,3-amino alcohols. Preliminary mechanism studies revealed that this intramolecular aminoperfluoroalkylation reaction initiated with the addition of a perfluoroalkyl radical to the terminal alkene, and the subsequent functionalization with the benzimidate motif via intramolecular substitution generated 1,3-oxazine products.

| [1] | Hu, J.; Ding, K . Acta Chim. Sinica 2018, 76, 905 (in Chinese). |
| [1] | ( 胡金波, 丁奎岭, 化学学报 , 2018, 76, 905.) |
| [2] | (a) Smart, B. E . J. Flurorine Chem. 2001, 109, 3 |
| [2] | (b) Hagmann, W. K . J. Med. Chem. 2008, 51, 4359; |
| [2] | (c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320; |
| [2] | (d) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422. |
| [3] | (a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257 |
| [3] | (b) Meyer, F. Chem. Commun. 2016, 52, 3077. |
| [4] | Tian, Y.; Chen, S.; Gu, Q.-S.; Lin, J.-S.; Liu, X.-Y . Tetrahedron Lett. 2018, 59, 203. |
| [5] | (a) Takamasa, F.; Yoshiko, S.; Hisao, U . Chem. Lett. 1987, 16, 521 |
| [5] | (b) Kim, E.; Choi, S.; Kim, H.; Cho, E. J. Chem.-Eur. J. 2013, 19, 6209; |
| [5] | (c) Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 11960; |
| [5] | (d) Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464; |
| [5] | (e) Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906. |
| [6] | For copper catalyzed intramolecular aminoperfuoroalkylation, see: (a) Egami, H.; Kawamura, S.; Miyazaki, A.; Sodeoka, M. Angew. Chem., Int. Ed. 2013, 52, 7841 |
| [6] | (b) Kawamura, S.; Egami, H.; Sodeoka, M. J. Am. Chem. Soc. 2015, 137, 4865; |
| [6] | (c) Kawamura, S.; Dosei, K.; Valverde, E.; Ushida, K.; Sodeoka, M . J. Org. Chem. 2017, 82, 12539; |
| [6] | (d) Lin, J.-S.; Liu, X.-G.; Zhu, X.-L.; Tan, B.; Liu, X.-Y . J. Org. Chem. 2014, 79, 7084; |
| [6] | (e) Lin, J.-S.; Xiong, Y.-P.; Ma, C.-L.; Zhao, L.-J.; Tan, B.; Liu, X.-Y . Chem.-Eur. J. 2014, 20, 1332; |
| [6] | (f) Li, X.-F.; Lin, J.-S.; Liu, X.-Y . Synthesis 2017, 49, 4213; |
| [6] | (g) Shen, K.; Wang, Q . Org. Chem. Front. 2016, 3, 222; |
| [6] | (h) Yu, L.-Z.; Wei, Y.; Shi, M . Chem. Commun. 2016, 52, 13163; |
| [6] | (i) Zhang, H.-Y.; Huo, W.; Ge, C.; Zhao, J.; Zhang, Y . Synlett 2017, 28, 962; |
| [6] | (j) Chang, B.; Su, Y.; Huang, D.; Wang, K.-H.; Zhang, W.; Shi, Y.; Zhang, X.; Hu, Y . J. Org. Chem. 2018, 83, 4365. |
| [7] | For enantioselective aminotrifluoromethylation of alkene, see: (a) Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357 |
| [7] | (b) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y . Nat. Commun. 2017, 8, 14841. |
| [8] | For recent examples of using imidates as nucleophile, see: (a) Brindle, C. S.; Yeung, C. S.; Jacobsen, E. N. Chem. Sci. 2013, 4, 2100 |
| [8] | (b) Zhu, R.; Yu, K.; Gu, Z. Org. Biomol. Chem. 2014, 12, 6653. |
| [9] | Mou, X.-Q.; Chen, X.-Y.; Chen, G.; He, G . Chem. Commun. 2018, 54, 515. |
| [10] | For selected examples of intramolecular C-H amination of imidates by other groups, see: (a) Wappes, E. A.; Nakafuku, K. M.; Nagib, D. A. J. Am. Chem. Soc. 2017, 139, 10204 |
| [10] | (b) Stateman, L. M.; Wappes, E. A.; Nakafuku, K. M.; Edwards, K. M.; Nagib, D. A. Chem. Sci. 2019, 10, 2693; |
| [10] | (c) Shaw, M.; Kumar, A. Org. Lett. 2019, 21, 3108. |
| [11] | Mou, X.-Q.; Rong, F.-M.; Zhang, H.; Chen, G.; He, G . Org. Lett. 2019, 21, 4657. |
| [12] | (a) Eisenberger, P.; Gischig, S.; Togni, A . Chem.-Eur. J. 2006, 12, 2579 |
| [12] | (b) Matou?ek, V.; Pietrasiak, E.; Schwenk, R.; Togni, A. J. Org. Chem. 2013, 78, 6763; |
| [12] | (c) Charpentier, J.; Früh, N.; Togni, A . Chem. Rev. 2015, 115, 650. |
| [13] | For selected reviews on the synthesis and application of perfluoroalkyl iodides, see: (a) Huang, B . Chin. J. Org. Chem. 1981, 1, 403 (in Chinese). |
| [13] | ( 黄炳南 , 有机化学, 1981, 1, 403.); |
| [13] | (b) Brace, N. O . J. Flurorine Chem. 1999, 93, 1; |
| [13] | (c) Brace, N. O . J. Flurorine Chem. 1999, 96, 101; |
| [13] | (d) Brace, N. O . J. Flurorine Chem. 2001, 108, 147; |
| [13] | (e) Murphy, P. M.; Baldwin, C. S.; Buck, R. C . J. Flurorine Chem. 2012, 138, 3; |
| [13] | (f) Huang, H.; Wang, X.; Wang, J . Chin. J. Org. Chem. 2019, 39, 1 (in Chinese). |
| [13] | ( 黄航, 王兮, 王剑波, 有机化学, 2019, 39, 1.) |
| [14] | (a) Chen, Q.-Y.; Chen, Y.-X.; Huang, W.-Y. Acta Chim. Sinica 1984, 42, 906 (in Chinese). |
| [14] | ( 陈庆云, 陈亚雄, 黄维垣, 化学学报 , 1984, 42, 906.); |
| [14] | (b) Chen, Q.-Y.; Yang, Z.-Y . J. Flurorine Chem. 1985, 28, 399; |
| [14] | (c) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1073 (in Chinese). |
| [14] | (陈庆云, 杨震宇, 化学学报, 1985, 43, 1073.); |
| [14] | (d) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1118 (in Chinese). |
| [14] | (陈庆云, 杨震宇, 化学学报, 1985, 43, 1118.); |
| [14] | (e) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 265 (in Chinese). |
| [14] | (陈庆云, 杨震宇, 化学学报, 1986, 44, 265.); |
| [14] | (f) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 1025 (in Chinese). |
| [14] | (陈庆云, 杨震宇, 化学学报, 1986, 44, 1025.); |
| [14] | (g) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1987, 45, 354 (in Chinese). |
| [14] | (陈庆云, 裘再明, 化学学报, 1987, 45, 354.); |
| [14] | (h) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1988, 46, 258 (in Chinese). |
| [14] | (陈庆云, 裘再明, 化学学报, 1988, 46, 258.); |
| [14] | (i) Chen, Q.-Y.; Chen, J.-G . Acta Chim. Sinica 1988, 46, 301 (in Chinese). |
| [14] | (陈庆云, 杨建国, 化学学报, 1988, 46, 301.); |
| [14] | (j) Xiao, Z.; Hu, H.; Ma, J.; Chen, Q.; Guo, Y . Chin. J. Chem. 2013, 31, 939; |
| [14] | (k) Su, Z.; Guo, Y.; Chen, Q.-Y.; Zhao, Z.-G.; Nian, B-Y . Chin. J. Chem. 2019, 37, 597. |
| [15] | For selected examples of perfluoroalkylation of aromatic compounds with perfluoroalkyl iodides, see (a) Iqbal, N.; Choi, S.; Ko, E.; Cho, E. J. Tetrahedron Lett. 2012, 53, 2005 |
| [15] | (b) Barata-Vallejo, S.; Flesia, M. M.; Lanta?o, B.; Argüello, J. E.; Pe?é?ory, A. B.; Postigo, A. Eur. J. Org. Chem. 2013, 2013, 998; |
| [15] | (c) Straathof, N. J. W.; Gemoets, H. P. L.; Wang, X.; Schouten, J. C.; Hessel, V.; No?l, T . ChemSusChem 2014, 7, 1612; |
| [15] | (d) Huang, Y.; Lei, Y.-Y.; Zhao, L.; Gu, J.; Yao, Q.; Wang, Z.; Li, X.-F.; Zhang, X.; He, C.-Y . Chem. Commun. 2018, 54, 13662; |
| [15] | (e) Yerien, D. E.; Cooke, M. V.; García Vior, M. C.; Barata- Vallejo, S.; Postigo, A . Org. Biomol. Chem. 2019, 17, 3741. |
| [16] | For selected examples of perfluoroalkylation of alkene with perfluoroalkyl iodides under visible light irradiation, see (a) Brace, N. O. J. Org. Chem. 1963, 28, 3093 |
| [16] | (b) Habib, M. H.; Mallouk, T. E. J. Flurorine Chem. 1991, 53, 53; |
| [16] | (c) Ogawa, A.; Imura, M.; Kamada, N.; Hirao, T . Tetrahedron Lett. 2001, 42, 2489; |
| [16] | (d) Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A . J. Org. Chem. 2004, 69, 6658; |
| [16] | (e) Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J . J. Am. Chem. Soc. 2012, 134, 8875; |
| [16] | (f) Mizuta, S.; Verhoog, S.; Engle, K. M.; Khotavivattana, T.; O’Duill, M.; Wheelhouse, K.; Rassias, G.; Médebielle, M.; Gouverneur, V . J. Am. Chem. Soc. 2013, 135, 2505; |
| [16] | (g) Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G . Org. Lett. 2017, 19, 1442; |
| [16] | (h) Beniazza, R.; Remisse, L.; Jardel, D.; Lastécouères, D.; Vincent, J.-M . Chem. Commun. 2018, 54, 7451; |
| [16] | (j) Rawner, T.; Lutsker, E.; Kaiser, C. A.; Reiser, O . ACS Catal. 2018, 8, 3950. |
| [17] | For selected examples of transition metal catalyzed perfluoroalkylation of alkene with perfluoroalkyl iodides, see: (a) Gil-Rubio, J.; Guerrero-Leal, J.; Blaya, M.; Vicente, J.; Bautista, D.; Jones, P. G . Organometallics 2012, 31, 1287 |
| [17] | (b) Blaya, M.; Bautista, D.; Gil- Rubio, J.; Vicente, J . Organometallics 2017, 36, 1245; |
| [17] | (c) Zheng, J.; Chen, P.; Yuan, Y.; Cheng, J . J. Org. Chem. 2017, 82, 5790. |
| [18] | For selected reviews on the synthesis and application of 1, 3-oxazines, see: (a) Schmidt, R. R .Synthesis 1972, 1972, 333 |
| [18] | (b) Sato, M.; Sunami, S.; Kaneko, C . Heterocycles 1996, 42, 861. |
| [19] | In the reaction of O-homoallyl benzimidates equipped with multi- substituted alkene and trichloroacetimidate analogue of 1, no desired product was detected, and most of the starting material remain unconsumed. |
/
| 〈 |
|
〉 |