化学学报 ›› 2019, Vol. 77 ›› Issue (9): 884-888.DOI: 10.6023/A19060220 上一篇 下一篇
所属专题: 有机自由基化学; 纪念南开大学化学学科创建100周年
研究通讯
投稿日期:2019-06-18
发布日期:2019-08-13
通讯作者:
陈弓,何刚
E-mail:gongchen@nankai.edu.cn;hegang@nankai.edu.cn
基金资助:
Zhang, Heng, Mou, Xueqing, Chen, Gong*( ), He, Gang*(
), He, Gang*( )
)
Received:2019-06-18
Published:2019-08-13
Contact:
Chen, Gong,He, Gang
E-mail:gongchen@nankai.edu.cn;hegang@nankai.edu.cn
Supported by:文章分享
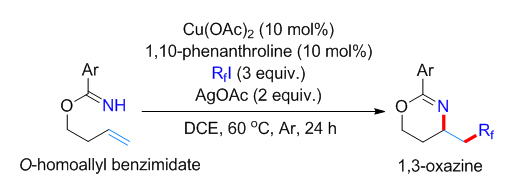
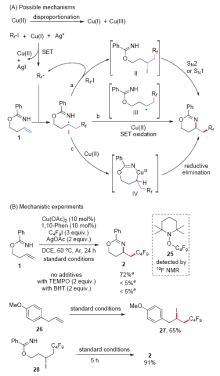
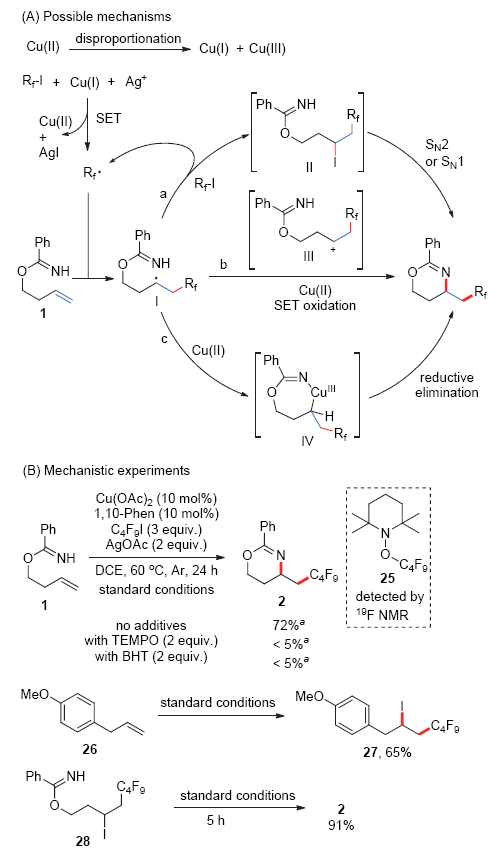
报道了铜催化苯甲酰亚胺高烯丙酯底物的分子内胺化全氟烷基化反应. 该反应以全氟碘代烷为全氟烷基化试剂, 醋酸铜为催化剂, 邻菲啰啉为配体, 在醋酸银存在下以中等的收率实现苯甲酰亚胺高烯丙酯底物末端双键的胺化全氟烷基化, 最终生成1,3-噁嗪类分子. 多种官能团取代的苯甲酰亚胺高烯丙酯和具有不同碳链长度的全氟碘代烷烃都能适用于该反应, 为多氟烷基取代的1,3-噁嗪类化合物的合成提供了一种简洁的方法. 多氟烷基取代的1,3-噁嗪类化合物还可在温和条件下高效转化为γ氨基醇衍生物. 初步的机理研究证明该反应经历了全氟烷基自由基对碳碳双键的亲电加成, 之后苯甲酰亚胺基团作为分子内亲核性胺源经历分子内亲核取代途径生成1,3-噁嗪骨架.
张衡, 牟学清, 陈弓, 何刚. 铜催化苯甲酰亚胺高烯丙酯的分子内胺化全氟烷基化反应[J]. 化学学报, 2019, 77(9): 884-888.
Zhang, Heng, Mou, Xueqing, Chen, Gong, He, Gang. Copper-catalyzed Intramolecular Aminoperfluoroalkylation Reaction of O-Homoallyl Benzimidates[J]. Acta Chimica Sinica, 2019, 77(9): 884-888.
| Entry | Catalyst | Additive | Solvent | Yielda/% of 2 |
|---|---|---|---|---|
| 1b | Cu(CH3CN)4PF6 | BOX ligand | DCE | NR |
| 2 | Cu(CH3CN)4PF6 | AgOAc | DCE | 44 |
| 3 | CuCl | AgOAc | DCE | 54 |
| 4 | CuBr | AgOAc | DCE | 62 |
| 5 | CuI | AgOAc | DCE | 60 |
| 6 | Cu(acac)2 | AgOAc | DCE | 54 |
| 7 | Cu(OTf)2 | AgOAc | DCE | 45 |
| 8 | Cu(OAc)2 | AgOAc | DCE | 72 (64)c |
| 9 | Cu(OAc)2 | AgOTFA | DCE | 19 |
| 10 | Cu(OAc)2 | AgOTf | DCE | 47 |
| 11 | Cu(OAc)2 | Ag2CO3 | DCE | 53 |
| 12 | Cu(OAc)2 | Cs2CO3 | DCE | 20 |
| 13 | Cu(OAc)2 | AgOAc | CH3CN | 67 |
| 14 | Cu(OAc)2 | AgOAc | THF | 52 |
| 15 | Cu(OAc)2 | AgOAc | EtOAc | 63 |
| 16 | Cu(OAc)2 | — | DCE | NR |
| 17 | AgOAc | DCE | <5 | |
| 18d | Cu(OAc)2 | AgOAc | DCE | <5 |
| 19e | Cu(OAc)2 | AgOAc | DCE | 53 |
| Entry | Catalyst | Additive | Solvent | Yielda/% of 2 |
|---|---|---|---|---|
| 1b | Cu(CH3CN)4PF6 | BOX ligand | DCE | NR |
| 2 | Cu(CH3CN)4PF6 | AgOAc | DCE | 44 |
| 3 | CuCl | AgOAc | DCE | 54 |
| 4 | CuBr | AgOAc | DCE | 62 |
| 5 | CuI | AgOAc | DCE | 60 |
| 6 | Cu(acac)2 | AgOAc | DCE | 54 |
| 7 | Cu(OTf)2 | AgOAc | DCE | 45 |
| 8 | Cu(OAc)2 | AgOAc | DCE | 72 (64)c |
| 9 | Cu(OAc)2 | AgOTFA | DCE | 19 |
| 10 | Cu(OAc)2 | AgOTf | DCE | 47 |
| 11 | Cu(OAc)2 | Ag2CO3 | DCE | 53 |
| 12 | Cu(OAc)2 | Cs2CO3 | DCE | 20 |
| 13 | Cu(OAc)2 | AgOAc | CH3CN | 67 |
| 14 | Cu(OAc)2 | AgOAc | THF | 52 |
| 15 | Cu(OAc)2 | AgOAc | EtOAc | 63 |
| 16 | Cu(OAc)2 | — | DCE | NR |
| 17 | AgOAc | DCE | <5 | |
| 18d | Cu(OAc)2 | AgOAc | DCE | <5 |
| 19e | Cu(OAc)2 | AgOAc | DCE | 53 |
| [1] |
Hu, J.; Ding, K . Acta Chim. Sinica 2018, 76, 905 (in Chinese).
doi: 10.6023/A1812E001 |
|
( 胡金波, 丁奎岭, 化学学报 , 2018, 76, 905.)
doi: 10.6023/A1812E001 |
|
| [2] |
(a) Smart, B. E . J. Flurorine Chem. 2001, 109, 3
doi: 10.1021/jm800219f |
|
(b) Hagmann, W. K . J. Med. Chem. 2008, 51, 4359;
doi: 10.1021/jm800219f |
|
|
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320;
doi: 10.1021/jm800219f |
|
|
(d) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
doi: 10.1021/jm800219f |
|
| [3] |
(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257
doi: 10.1021/jm501100b |
|
(b) Meyer, F. Chem. Commun. 2016, 52, 3077.
doi: 10.1021/jm501100b |
|
| [4] |
Tian, Y.; Chen, S.; Gu, Q.-S.; Lin, J.-S.; Liu, X.-Y . Tetrahedron Lett. 2018, 59, 203.
doi: 10.1016/j.tetlet.2017.12.034 |
| [5] |
(a) Takamasa, F.; Yoshiko, S.; Hisao, U . Chem. Lett. 1987, 16, 521
doi: 10.1246/cl.1987.521 |
|
(b) Kim, E.; Choi, S.; Kim, H.; Cho, E. J. Chem.-Eur. J. 2013, 19, 6209;
doi: 10.1246/cl.1987.521 |
|
|
(c) Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2014, 53, 11960;
doi: 10.1246/cl.1987.521 |
|
|
(d) Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464;
doi: 10.1246/cl.1987.521 |
|
|
(e) Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906.
doi: 10.1246/cl.1987.521 |
|
| [6] |
For copper catalyzed intramolecular aminoperfuoroalkylation, see: (a) Egami, H.; Kawamura, S.; Miyazaki, A.; Sodeoka, M. Angew. Chem., Int. Ed. 2013, 52, 7841
doi: 10.1002/anie.v52.30 |
|
(b) Kawamura, S.; Egami, H.; Sodeoka, M. J. Am. Chem. Soc. 2015, 137, 4865;
doi: 10.1002/anie.v52.30 |
|
|
(c) Kawamura, S.; Dosei, K.; Valverde, E.; Ushida, K.; Sodeoka, M . J. Org. Chem. 2017, 82, 12539;
doi: 10.1002/anie.v52.30 |
|
|
(d) Lin, J.-S.; Liu, X.-G.; Zhu, X.-L.; Tan, B.; Liu, X.-Y . J. Org. Chem. 2014, 79, 7084;
doi: 10.1002/anie.v52.30 |
|
|
(e) Lin, J.-S.; Xiong, Y.-P.; Ma, C.-L.; Zhao, L.-J.; Tan, B.; Liu, X.-Y . Chem.-Eur. J. 2014, 20, 1332;
doi: 10.1002/anie.v52.30 |
|
|
(f) Li, X.-F.; Lin, J.-S.; Liu, X.-Y . Synthesis 2017, 49, 4213;
doi: 10.1002/anie.v52.30 |
|
|
(g) Shen, K.; Wang, Q . Org. Chem. Front. 2016, 3, 222;
doi: 10.1002/anie.v52.30 |
|
|
(h) Yu, L.-Z.; Wei, Y.; Shi, M . Chem. Commun. 2016, 52, 13163;
doi: 10.1002/anie.v52.30 |
|
|
(i) Zhang, H.-Y.; Huo, W.; Ge, C.; Zhao, J.; Zhang, Y . Synlett 2017, 28, 962;
doi: 10.1002/anie.v52.30 |
|
|
(j) Chang, B.; Su, Y.; Huang, D.; Wang, K.-H.; Zhang, W.; Shi, Y.; Zhang, X.; Hu, Y . J. Org. Chem. 2018, 83, 4365.
doi: 10.1002/anie.v52.30 |
|
| [7] |
For enantioselective aminotrifluoromethylation of alkene, see: (a) Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357
doi: 10.1021/jacs.6b04077 |
|
(b) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y . Nat. Commun. 2017, 8, 14841.
doi: 10.1021/jacs.6b04077 |
|
| [8] |
For recent examples of using imidates as nucleophile, see: (a) Brindle, C. S.; Yeung, C. S.; Jacobsen, E. N. Chem. Sci. 2013, 4, 2100
doi: 10.1039/c3sc50410g |
|
(b) Zhu, R.; Yu, K.; Gu, Z. Org. Biomol. Chem. 2014, 12, 6653.
doi: 10.1039/c3sc50410g |
|
| [9] |
Mou, X.-Q.; Chen, X.-Y.; Chen, G.; He, G . Chem. Commun. 2018, 54, 515.
doi: 10.1039/C7CC08897C |
| [10] |
For selected examples of intramolecular C-H amination of imidates by other groups, see: (a) Wappes, E. A.; Nakafuku, K. M.; Nagib, D. A. J. Am. Chem. Soc. 2017, 139, 10204
doi: 10.1021/jacs.7b05214 |
|
(b) Stateman, L. M.; Wappes, E. A.; Nakafuku, K. M.; Edwards, K. M.; Nagib, D. A. Chem. Sci. 2019, 10, 2693;
doi: 10.1021/jacs.7b05214 |
|
|
(c) Shaw, M.; Kumar, A. Org. Lett. 2019, 21, 3108.
doi: 10.1021/jacs.7b05214 |
|
| [11] |
Mou, X.-Q.; Rong, F.-M.; Zhang, H.; Chen, G.; He, G . Org. Lett. 2019, 21, 4657.
doi: 10.1021/acs.orglett.9b01552 |
| [12] |
(a) Eisenberger, P.; Gischig, S.; Togni, A . Chem.-Eur. J. 2006, 12, 2579
doi: 10.1002/(ISSN)1521-3765 |
|
(b) Matoušek, V.; Pietrasiak, E.; Schwenk, R.; Togni, A. J. Org. Chem. 2013, 78, 6763;
doi: 10.1002/(ISSN)1521-3765 |
|
|
(c) Charpentier, J.; Früh, N.; Togni, A . Chem. Rev. 2015, 115, 650.
doi: 10.1002/(ISSN)1521-3765 |
|
| [13] |
For selected reviews on the synthesis and application of perfluoroalkyl iodides, see: (a) Huang, B . Chin. J. Org. Chem. 1981, 1, 403 (in Chinese).
doi: 10.6023/cjoc201808030 |
|
( 黄炳南 , 有机化学, 1981, 1, 403.);
doi: 10.6023/cjoc201808030 |
|
|
(b) Brace, N. O . J. Flurorine Chem. 1999, 93, 1;
doi: 10.6023/cjoc201808030 |
|
|
(c) Brace, N. O . J. Flurorine Chem. 1999, 96, 101;
doi: 10.6023/cjoc201808030 |
|
|
(d) Brace, N. O . J. Flurorine Chem. 2001, 108, 147;
doi: 10.6023/cjoc201808030 |
|
|
(e) Murphy, P. M.; Baldwin, C. S.; Buck, R. C . J. Flurorine Chem. 2012, 138, 3;
doi: 10.6023/cjoc201808030 |
|
|
(f) Huang, H.; Wang, X.; Wang, J . Chin. J. Org. Chem. 2019, 39, 1 (in Chinese).
doi: 10.6023/cjoc201808030 |
|
|
( 黄航, 王兮, 王剑波, 有机化学, 2019, 39, 1.)
doi: 10.6023/cjoc201808030 |
|
| [14] |
(a) Chen, Q.-Y.; Chen, Y.-X.; Huang, W.-Y. Acta Chim. Sinica 1984, 42, 906 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
( 陈庆云, 陈亚雄, 黄维垣, 化学学报 , 1984, 42, 906.);
doi: 10.1002/cjoc.v31.7 |
|
|
(b) Chen, Q.-Y.; Yang, Z.-Y . J. Flurorine Chem. 1985, 28, 399;
doi: 10.1002/cjoc.v31.7 |
|
|
(c) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1073 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1985, 43, 1073.);
doi: 10.1002/cjoc.v31.7 |
|
|
(d) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1985, 43, 1118 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1985, 43, 1118.);
doi: 10.1002/cjoc.v31.7 |
|
|
(e) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 265 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1986, 44, 265.);
doi: 10.1002/cjoc.v31.7 |
|
|
(f) Chen, Q.-Y.; Yang, Z.-Y . Acta Chim. Sinica 1986, 44, 1025 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨震宇, 化学学报, 1986, 44, 1025.);
doi: 10.1002/cjoc.v31.7 |
|
|
(g) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1987, 45, 354 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 裘再明, 化学学报, 1987, 45, 354.);
doi: 10.1002/cjoc.v31.7 |
|
|
(h) Chen, Q.-Y.; Qiu, Z.-M . Acta Chim. Sinica 1988, 46, 258 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 裘再明, 化学学报, 1988, 46, 258.);
doi: 10.1002/cjoc.v31.7 |
|
|
(i) Chen, Q.-Y.; Chen, J.-G . Acta Chim. Sinica 1988, 46, 301 (in Chinese).
doi: 10.1002/cjoc.v31.7 |
|
|
(陈庆云, 杨建国, 化学学报, 1988, 46, 301.);
doi: 10.1002/cjoc.v31.7 |
|
|
(j) Xiao, Z.; Hu, H.; Ma, J.; Chen, Q.; Guo, Y . Chin. J. Chem. 2013, 31, 939;
doi: 10.1002/cjoc.v31.7 |
|
|
(k) Su, Z.; Guo, Y.; Chen, Q.-Y.; Zhao, Z.-G.; Nian, B-Y . Chin. J. Chem. 2019, 37, 597.
doi: 10.1002/cjoc.v31.7 |
|
| [15] |
For selected examples of perfluoroalkylation of aromatic compounds with perfluoroalkyl iodides, see (a) Iqbal, N.; Choi, S.; Ko, E.; Cho, E. J. Tetrahedron Lett. 2012, 53, 2005
doi: 10.1016/j.tetlet.2012.02.032 |
|
(b) Barata-Vallejo, S.; Flesia, M. M.; Lantaño, B.; Argüello, J. E.; Peñéñory, A. B.; Postigo, A. Eur. J. Org. Chem. 2013, 2013, 998;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(c) Straathof, N. J. W.; Gemoets, H. P. L.; Wang, X.; Schouten, J. C.; Hessel, V.; Noël, T . ChemSusChem 2014, 7, 1612;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(d) Huang, Y.; Lei, Y.-Y.; Zhao, L.; Gu, J.; Yao, Q.; Wang, Z.; Li, X.-F.; Zhang, X.; He, C.-Y . Chem. Commun. 2018, 54, 13662;
doi: 10.1016/j.tetlet.2012.02.032 |
|
|
(e) Yerien, D. E.; Cooke, M. V.; García Vior, M. C.; Barata- Vallejo, S.; Postigo, A . Org. Biomol. Chem. 2019, 17, 3741.
doi: 10.1016/j.tetlet.2012.02.032 |
|
| [16] |
For selected examples of perfluoroalkylation of alkene with perfluoroalkyl iodides under visible light irradiation, see (a) Brace, N. O. J. Org. Chem. 1963, 28, 3093
doi: 10.1021/jo01046a039 |
|
(b) Habib, M. H.; Mallouk, T. E. J. Flurorine Chem. 1991, 53, 53;
doi: 10.1021/jo01046a039 |
|
|
(c) Ogawa, A.; Imura, M.; Kamada, N.; Hirao, T . Tetrahedron Lett. 2001, 42, 2489;
doi: 10.1021/jo01046a039 |
|
|
(d) Tsuchii, K.; Imura, M.; Kamada, N.; Hirao, T.; Ogawa, A . J. Org. Chem. 2004, 69, 6658;
doi: 10.1021/jo01046a039 |
|
|
(e) Wallentin, C.-J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. J . J. Am. Chem. Soc. 2012, 134, 8875;
doi: 10.1021/jo01046a039 |
|
|
(f) Mizuta, S.; Verhoog, S.; Engle, K. M.; Khotavivattana, T.; O’Duill, M.; Wheelhouse, K.; Rassias, G.; Médebielle, M.; Gouverneur, V . J. Am. Chem. Soc. 2013, 135, 2505;
doi: 10.1021/jo01046a039 |
|
|
(g) Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G . Org. Lett. 2017, 19, 1442;
doi: 10.1021/jo01046a039 |
|
|
(h) Beniazza, R.; Remisse, L.; Jardel, D.; Lastécouères, D.; Vincent, J.-M . Chem. Commun. 2018, 54, 7451;
doi: 10.1021/jo01046a039 |
|
|
(j) Rawner, T.; Lutsker, E.; Kaiser, C. A.; Reiser, O . ACS Catal. 2018, 8, 3950.
doi: 10.1021/jo01046a039 |
|
| [17] |
For selected examples of transition metal catalyzed perfluoroalkylation of alkene with perfluoroalkyl iodides, see: (a) Gil-Rubio, J.; Guerrero-Leal, J.; Blaya, M.; Vicente, J.; Bautista, D.; Jones, P. G . Organometallics 2012, 31, 1287
doi: 10.1021/om2009588 |
|
(b) Blaya, M.; Bautista, D.; Gil- Rubio, J.; Vicente, J . Organometallics 2017, 36, 1245;
doi: 10.1021/om2009588 |
|
|
(c) Zheng, J.; Chen, P.; Yuan, Y.; Cheng, J . J. Org. Chem. 2017, 82, 5790.
doi: 10.1021/om2009588 |
|
| [18] |
For selected reviews on the synthesis and application of 1, 3-oxazines, see: (a) Schmidt, R. R .Synthesis 1972, 1972, 333
doi: 10.1055/s-1972-21882 |
|
(b) Sato, M.; Sunami, S.; Kaneko, C . Heterocycles 1996, 42, 861.
doi: 10.1055/s-1972-21882 |
|
| [19] | In the reaction of O-homoallyl benzimidates equipped with multi- substituted alkene and trichloroacetimidate analogue of 1, no desired product was detected, and most of the starting material remain unconsumed. |
| [1] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [2] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [3] | 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63. |
| [4] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| [5] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| [6] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [7] | 张荣华, 许冰, 张展鸣, 张俊良. Ming-Phos/铜催化的亚甲胺叶立德与硝基烯烃的不对称[3+2]环加成反应[J]. 化学学报, 2020, 78(3): 245-249. |
| [8] | 黄浩, 林华鑫, 王敏, 廖建. 1,2-苯基异噁唑为氮源的铜催化苯乙烯不对称硼胺化[J]. 化学学报, 2020, 78(11): 1229-1234. |
| [9] | 梁欢, 苟阿龙, 高珠鹏, 雷林生, 王博文, 余兰, 徐学涛, 王少华. 铜催化的α-氨基丙二腈的脱氰氧代反应:一种合成叔酰胺的新方法[J]. 化学学报, 2020, 78(10): 1064-1068. |
| [10] | 林凤闺蓉, 梁宇杰, 郦鑫耀, 宋颂, 焦宁. 氧气氧化铜催化的苯胺邻位叠氮化反应[J]. 化学学报, 2019, 77(9): 906-910. |
| [11] | 成忠明, 陈品红, 刘国生. 光/铜共催化远程C—H键的不对称氰基化反应[J]. 化学学报, 2019, 77(9): 856-860. |
| [12] | 李雪飞, 林进顺, 王建, 李忠良, 顾强帅, 刘心元. 铜/手性磷酸催化烯烃不对称自由基胺芳基化[J]. 化学学报, 2018, 76(11): 878-882. |
| [13] | 张涌灵, 王敏, 曹鹏, 廖建. 铜催化苯乙烯不对称硼胺化反应[J]. 化学学报, 2017, 75(8): 794-797. |
| [14] | 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武. 2H-吡咯的简易合成方法:金催化与路易斯酸催化的组合应用[J]. 化学学报, 2016, 74(1): 49-53. |
| [15] | 李森, 韩静, 李昂. 中断的Fisher吲哚合成法及其在生物碱合成中的应用[J]. 化学学报, 2013, 71(03): 295-298. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||