

可见光催化的氮自由基导向远程sp 3碳氢官能团化反应
收稿日期: 2019-05-15
网络出版日期: 2019-08-14
Visible-Light-Induced N-Radical Directed Remote Functionalization of sp 3 C-H Bonds
Received date: 2019-05-15
Online published: 2019-08-14
肖丽 , 李嘉恒 , 王挺 . 可见光催化的氮自由基导向远程sp 3碳氢官能团化反应[J]. 化学学报, 2019 , 77(9) : 841 -849 . DOI: 10.6023/A19050183
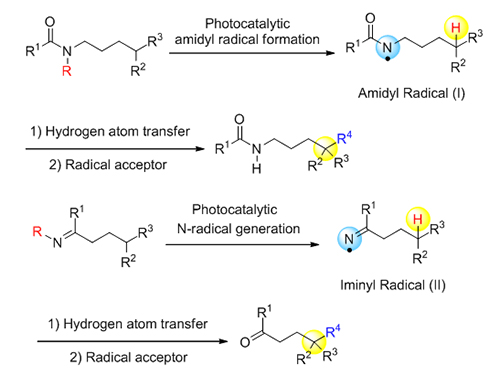
The selective functionalization of unactivated sp 3 carbon-hydrogen (C—H) bond is an attractive strategy in modern organic transformation. The hydrogen atom transfer (HAT) catalysis has recently shown its advances in remotely selective activation of an inert C—H bond with great functional group compatibility, generating new carbon-carbon (C—C) bonds and carbon-heteroatom (C—O, C—N, C—X) bonds. Therefore, the remote sp 3 C—H functionalization has become an intensively investigated research area, drawing extensive attention in synthetic community. Particularly, the 1,5-hydrogen atom abstraction of nitrogen radicals, the key step of the Hoffman-L?ffler-Freytag (HLF) reaction, has been widely applied in the preparation of heterocycles. Comparing to the well-studied area of nucleophilic N-species, N-centered radical based reactions are still underdeveloped. The limited utility is partially due to the required use of hazardous radical initiators, elevated temperatures, or high-energy UV irradiation for the generation of N-radicals. Recently, visible-light photoredox catalysis has been leading efficient accesses to Nitrogen-radicals under mild conditions. The visible-light-induced nitrogen radical formation has also stimulated the development of the remote sp 3 C—H functionalization by photoredox catalysis. The classic HLF reaction requires pre-functionalization at N-center in the substrate to promote the formation of N-radical. Recently, a direct N—H single electron transfer (SET) oxidation was realized by photoredox catalysis in Knowles and Rovis’s group, generating N-radicals efficiently. The processes significantly simplified the preparation of the HLF reaction substrates and broaden the application of this classic reaction. In addition, the visible-light-induced nitrogen radical-directed reaction on modified imines provided possibilities for the remote sp 3 C—H functionalization of ketones, as ketone is the product of imine hydrolysis. Moreover, the application of chiral Lewis acid catalysis combined with visible-light photoredox catalysis enabled the asymmetric alkylation of the unactivated remote sp 3 C—H position, which achieves both regioselective and stereoselective functionalization. In conclusion, this strategy takes advantage of mild generation of N-radicals upon visible-light excitation. Subsequent 1,5-hydrogen atom transfer (1,5-HAT) and intermolecular radical coupling would realize the remote functionalization of unactivated sp 3 C—H bonds. The strategies have been successfully applied in remote C(sp 3)—H amidation, fluorination, chlorination, iodination, alkylation, vinylation, allylation, oxygenation, thioetherification, cyanation and alkynylation. In this review, we focus on visible-light-induced nitrogen radical directed functionalization of remote sp 3 C—H bonds, summarized the methodologies, and briefly reviewed their synthetic applications in pharmaceuticals and natural products.
Key words: visible-light; radical; remote functionalization
| [1] | Prier C. K.; Rankic D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [2] | Hoplinson M. N.; Sahoo B.; Li J.-L.; Glorius F . Chem. -Eur. J. 2014, 20, 3874. |
| [3] | K?rk?s M. D.; Porco J. A. Jr.; Stephenson, C. R. J. Chem. Rev. 2016, 116, 9683. |
| [4] | Xuan J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828. |
| [5] | Narayanam J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102. |
| [6] | Yoon, T. P. ACS Catal. 2013, 3, 895. |
| [7] | Nicewicz D. A.; Nguyen T. M . ACS Catal. 2014, 4. 355. |
| [8] | Fukuzumi S.; Ohkubo, K. Org. Biomol. Chem. 2014, 12, 6059. |
| [9] | Hari D. P.; K?nig B . Chem. Commun. 2014, 50, 6688. |
| [10] | Martin M. L.; Santos-Juanes, L.; Arques, A.; Amat, A. M.; Miranda, M. A . Chem. Rev. 2012, 112, 1710. |
| [11] | Nicewicz D. A.; Romero, N. A. Chem. Rev. 2016, 116, 10075. |
| [12] | Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J . Chem. Soc. Rev. 2016, 45, 2044. |
| [13] | Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan, D. W. C. Nat. Rev. Chem. 2017, 1, 52. |
| [14] | Tellis J. C.; Kelly C. B.; Primer D. N.; Jouffroy M.; Patel N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429. |
| [15] | Chen Y.-Y.; Lu L.-Q.; Yu D.-G.; Zhu C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24. |
| [16] | He J.; Wasa M.; Chan K. S.; Shao Q.; Yu J.-Q . Chem. Rev. 2016, 117, 8754. |
| [17] | Gutekunst W. R.; Baran P. S . Chem. Soc. Rev. 2011, 40, 1976. |
| [18] | Yamaguchi J.; Yamaguchi A. D.; Itami K . Angew. Chem., Int. Ed. 2012, 51, 8960. |
| [19] | Huang Z.; Lim H. N.; Mo F.; Young M. C.; Dong G . Chem. Soc. Rev. 2015, 44, 7764. |
| [20] | (a) Feng J.; Li B.; Jiang J.; Zhang M.; Ouyang W.; Li C.; Fu Y.; Gu, Z. Chin. J. Chem. 2018, 36, 11. |
| [20] | (b) Pei P.; Zhang F.; Yi H.; Lei, A. Acta Chim. Sinica 2017, 75, 15. |
| [20] | (裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15.) |
| [20] | (c) Zhong, J.; Meng, Q.; Chen, B.; Tung, C.-H.; Wu, L.-Z . Acta Chim. Sinica. 2017, 75, 34. |
| [20] | (钟建基, 孟庆元, 陈彬, 佟振合, 吴骊珠, 化学学报, 2017, 75, 34.) |
| [21] | Chiba S.; Chen H . Org. Biomol. Chem. 2014, 12, 4051. |
| [22] | Robertson J.; Pillai J.; Lush, R. K. Chem. Soc. Rev. 2001, 30, 94. |
| [23] | Mayer J. M . Acc. Chem. Res. 2010, 44, 36. |
| [24] | Protti S.; Fagnoni M.; Ravelli D . ChemCatChem 2015, 7, 1516. |
| [25] | Wolff M. E . Chem. Rev. 1963, 63, 55. |
| [26] | Qin Q.; Yu S . Org. Lett. 2015, 17, 1894. |
| [27] | Choi G. J.; Zhu Q.; Miller D. C.; Gu C. J.; Knowles R. R . Nature 2016, 539, 268. |
| [28] | Chu J. C. K.; Rovis T . Nature 2016, 539, 272. |
| [29] | Chen D.-F.; Chu J. C. K.; Rovis, T. J. Am. Chem. Soc. 2017, 139, 14897. |
| [30] | Yuan W.; Zhou Z.; Gong L.; Meggers E . Chem. Commun. 2017, 53, 8964. |
| [31] | Shu W.; Nevado, C. Angew. Chem., Int. Ed. 2017, 56, 1881. |
| [32] | Chen H.; Guo L.; Yu S . Org. Lett. 2018, 20, 6255. |
| [33] | Shen X.; Zhao J.; Yu S . Org. Lett. 2018, 20, 5523. |
| [34] | Xia Y.; Wang L.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 12940. |
| [35] | Dauncey E. M.; Morcillo S. P.; Douglas J. J.; Sheikh N. S.; Leonori, D. Angew. Chem., Int. Ed. 2018, 57, 744. |
| [36] | Jiang H.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 1692. |
| [37] | Morcillo S. P.; Dauncey E. M.; Kim J. H.; Douglas J. J.; Sheikh N. S.; Leonori, D. Angew. Chem., Int. Ed. 2018, 57, 12945. |
| [38] | Wappes E. A.; Vanitcha A.; Nagib, D. A. Chem. Sci. 2018, 9, 4500. |
| [39] | Wu K.; Wang L.; Col?n-Rodr?guez S.; Flechsig G.; Wang, T. Angew. Chem., Int. Ed. 2019, 58, 1774. |
| [40] | Xu B.; Tambar, U. K. ACS Catal. 2019, 9, 4727. |
/
| 〈 |
|
〉 |