

点手性调控的三股铕螺旋体的非对映选择性自组装及圆偏振发光
收稿日期: 2021-04-28
网络出版日期: 2021-06-11
基金资助
国家自然科学基金(51773054); 国家自然科学基金(51872077); 国家自然科学基金(52073080)
Point Chirality Regulated Diastereoselective Self-Assembly and Circularly Polarized Luminescence in Eu(III) Triple-Stranded Helicates
Received date: 2021-04-28
Online published: 2021-06-11
Supported by
National Natural Science Foundation of China(51773054); National Natural Science Foundation of China(51872077); National Natural Science Foundation of China(52073080)
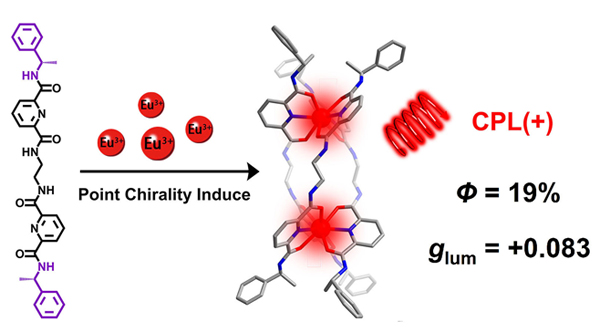
具有超分子手性的稀土螺旋体为合成高性能稀土圆偏振发光(CPL)材料提供了结构基础. 然而, 稀土Ln(III)离子较大的半径和不稳定的配位几何构型为合成高光学纯度的稀土螺旋体带来了困难和挑战. 本工作通过在双三齿配体端基引入点手性的方式成功构筑了一对儿对映体纯的手性双核三股铕螺旋体, [Eu2(LRR)3](OTf)6和[Eu2(LSS)3](OTf)6. 结合全面的光谱表征和半经验分子力学模型证实了配体端基的点手性成功诱导两个稀土Eu3+离子采取相同的Λ或Δ构型, 并形成具有单一M或P螺旋构象的螺旋体. 配合物的镜像圆二色(CD)和CPL光谱也进一步验证了一对儿光学纯对映体的形成. 手性光学性质研究显示螺旋体在乙腈中表现出适中的发光不对称因子(|glum|=0.083)和良好的发光量子产率(QY=19%). 该方法为制备性能优异的手性稀土CPL材料提供了一种可行性参考.
关键词: 稀土配合物; 手性螺旋体; 手性诱导; 圆偏振发光(CPL)
宋龙飞 , 周妍妍 , 高婷 , 闫鹏飞 , 李洪峰 . 点手性调控的三股铕螺旋体的非对映选择性自组装及圆偏振发光[J]. 化学学报, 2021 , 79(8) : 1042 -1048 . DOI: 10.6023/A21040185
The supramolecular chirality of lanthanide helicate offers a structural base to synthesize the excellent lanthanide circularly polarized luminescent (CPL) materials. However, the larger radii and the labile coordination geometries of the Ln(III) ions make it difficult to control the diastereoselectivity of lanthanide helicates in the self-assembly process. Herein, a pair of chiral dinuclear europium triple-stranded helicates [Eu2(LRR)3](OTf)6 and [Eu2(LSS)3](OTf)6 (LRR/SS=N,Nʹ-(ethane-4,4ʹ-diyl)bis[6-(R/S)-(1-phenethylcarbamoyl)-pyridine-2-dimethylamide]) were synthesized via point chirality induced strategy. The ligands LRR/SS were composed of two chiral 2,6-dipicolinic amides as coordination units and ethylenediamine moieties as spacers. General procedure for the preparation of ligands LRR/SS and corresponding europium complexes [Eu2(LRR/SS)3](OTf)6: 6-(Methoxycarbonyl)picolinic acid 4a (1.50 g, 5.6 mmol) was dissolved in 5 mL SOCl2 and stirred for 6 h. The white precipitate was obtained after removed SOCl2, and then it was added to 30 mL CH2Cl2 containing 4-dimethylaminopyridine (0.12 g, 1.0 mmol) and triethylamine (0.48 g, 8.0 mmol). Ethylenediamine (0.12 g, 2.0 mmol) in 5 mL CH2Cl2 was added by dropwise to the above solution and further stirred for 10 h. After the solution was washed with 1 mol•L-1 HCl, saturated sodium bicarbonate and water, dried over sodium sulfate, and evaporated to remove solvent. The crude product was purified by crystallization from CH2Cl2:n-hexane (V/V=1:20) to give LRR/SS (0.54 g, 47%). LRR/SS (0.10 g, 0.18 mmol) dissolved in 10 mL acetonitrile, Eu(OTf)3 (0.07 g, 0.12 mmol) in 5 mL acetonitrile was added dropwise to the above solution and stirred for 24 h. After the slow volatilization of reaction solution, the white crystals were obtained, [Eu2(LRR/SS)3](OTf)6 (0.12 g, 71%). Combination of the comprehensive spectral characteristics and semiempirical geometry optimization demonstrated that the point chirality at the terminal of the ligand successfully controlled the Δ or Λ configuration around the metal center and the P or M helical conformation of the helicates. The mirror-image circular dichroism (CD) and CPL spectra further confirmed the formation of the enantiomer pairs. Notably, the helicates exhibit excellent CPL emission with the |glum| values reaching to 0.083 and the modest luminescence quantum yields (QYs=19%) in CH3CN. This study provides an effective strategy for the syntheses of chiral lanthanide CPL materials with excellent performance.

| [1] | (a) LimD.-Y. J. Korean Phys. Soc. 2006, 49, S505. |
| [1] | (b) WernerE.J.; DattaA.; JocherC.J.; RaymondK.N. Angew. Chem., Int. Ed. 2008, 47, 8568. |
| [1] | (c) HuoS.; DuanP.; JiaoT.; PengQ.; LiuM. Angew. Chem., Int. Ed. 2017, 56, 12174. |
| [1] | (d) ZinnaF.; GiovanellaU.; Di BariL. Adv. Mater. 2015, 27, 1791. |
| [1] | (e) VictorT.W.; O'TooleK.H.; EasthonL.M.; GeM.; SmithR.J.; HuangX.; YanH.; ChuY.S.; ChenS.; GursoyD.; RalleM.; ImperialiB.; AllenK.N.; MillerL.M. J. Am. Chem. Soc. 2020, 142, 2145. |
| [2] | HuckN.P.M.; JagerW.F.; de LangeB.; FeringaB.L. Science 1996, 273, 1686. |
| [3] | (a) CarrR.; EvansN.H.; ParkerD. Chem. Soc. Rev. 2012, 41, 7673. |
| [3] | (b) ShuvaevS.; FoxM.A.; ParkerD. Angew. Chem., Int. Ed. 2018, 57, 7488. |
| [3] | (c) ShuvaevS.; SuturinaE.A.; MasonK; ParkerD. Chem. Sci. 2018, 9, 2996. |
| [3] | (d) StaszakK.; WieszczyckaK.; MarturanoV.; TylkowskiB. Coord. Chem. Rev. 2019, 397, 76. |
| [4] | (a) ZinnaF.; Di BariL. Chirality 2015, 27, 1. |
| [4] | (b) ShuvaevS.; StarckM.; ParkerD. Chem. - Eur. J. 2017, 23, 9974. |
| [4] | (c) YangY.; da CostaR.C.; FuchterM.J.; CampbellA.J. Nat. Photonics 2013, 7, 634. |
| [4] | (d) SethyR.; KumarJ.; MétivierR.; LouisM.; NakataniK.; MecheriN.M.T.; SubhakumariA.; ThomasK.G.; KawaiT.; NakashimaT. Angew. Chem., Int. Ed. 2017, 56, 15053. |
| [4] | (e) StaszakK.; WieszczyckaK.; MarturanoV.; TylkowskiB. Coord. Chem. Rev. 2019, 397, 76. |
| [4] | (f) ShuvaevS.; SuturinaE.A.; MasonK.; ParkerD. Chem. Sci. 2018, 9, 2996. |
| [5] | (a) ZhouY.; LiH.; ZhuT.; GaoT.; YanP. J. Am. Chem. Soc. 2019, 141, 19634. |
| [5] | (b) LiuD.; ZhouY.; ZhangY.; LiH.; ChenP.; SunW.; GaoT.; YanP. Inorg. Chem. 2018, 57, 8332. |
| [5] | (c) SugimotoM.; LiuX.-L.; TsunegaS.; NakajimaE.; AbeS.; NakashimaT.; KawaiT.; JinR.-H. Chem. - Eur. J. 2018, 24, 6519. |
| [5] | (d) TanY.B; OkayasuY.; KataoS.; NishikawaY.; AsanomaF.; YamadaM.; YuasaJ.; KawaiT. J. Am. Chem. Soc. 2020, 142, 17653. |
| [5] | (e) DengM.; SchleyN.D.; UngG. Chem. Commun. 2020, 56, 14813. |
| [5] | (f) HasegawaY.; MiuraY.; KitagawaY.; WadaS.; NakanishiT.; FushimiK.; SekiT.; ItoH.; IwasaT.; TaketsuguT.; GonM.; TanakaK.; ChujoY.; HattoriS.; KarasawaM.; IshiiK. Chem. Commun. 2018, 54, 10695. |
| [5] | (g) ZhangJ.; DaiL.; WebsterA.M.; ChanW.T.K.; MackenzieL.E.; PalR.; CobbS.L.; LawG.-L. Angew. Chem., Int. Ed. 2021, 60, 1004. |
| [6] | (a) LiM.; LinW.B.; FangL.; ChenC.F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese) |
| [6] | ( 李猛, 林伟彬, 房蕾, 陈传峰, 化学学报, 2017, 75, 1150.) |
| [6] | (b) ZhangL.; ZhaoW.L.; LiM.; LuH.Y.; ChenC.F. Acta Chim. Sinica 2020, 78, 1030. (in Chinese) |
| [6] | ( 张亮, 赵文龙, 李猛, 吕海燕, 陈传峰, 化学学报, 2020, 78, 1030.) |
| [6] | (c) SunZ.-B.; LiuJ.-K.; YuanD.-F.; ZhaoZ.-H.; ZhuX.-Z.; LiuD.-H.; PengQ.; ZhaoC.-H. Angew. Chem., Int. Ed. 2019, 58, 4840. |
| [6] | (d) JiangQ.; XuX.; YinP.-A.; MaK.; ZhenY.; DuanP.; PengQ.; ChenW.-Q.; DingB. J. Am. Chem. Soc. 2019, 141, 9490. |
| [6] | (e) TakaishiK.; YasuiM.; EmaT. J. Am. Chem. Soc. 2018, 140, 5334. |
| [6] | (f) FeuillastreS.; PautonM.; GaoL.; DesmarchelierA.; RiivesA.J.; PrimD.; TondelierD.; Ge?royB.; MullerG.; ClavierG.; PietersG. J. Am. Chem. Soc. 2016, 138, 3990. |
| [6] | (g) CruzC.M.; Castro-FernándezS.; MaçôasE.; CuervaJ.M.; CampañaA.G. Angew. Chem., Int. Ed. 2018, 57, 14782. |
| [6] | (h) LiuJ.G.; YinF.; HuJ.; JuY. Chin. J. Org. Chem. 2021, 41, 1031.. (in Chinese) |
| [6] | ( 刘金果, 殷凤, 胡君, 巨勇, 有机化学, 2021, 41, 1031.) |
| [7] | (a) San Jose, B.A.; Matsushita, S.; Akagi, K. J. Am. Chem. Soc. 2012, 134, 19795. |
| [7] | (b) San Jose, B.A.; Matsushita, S.; Akagi, K. J. Am. Chem. Soc. 2012, 134, 19795. |
| [7] | (c) San Jose, B.A.; Yan, J.; Akagi, K. Angew. Chem., Int. Ed. 2014, 53, 10641. |
| [7] | (d) LeeS.; KimK.Y.; JungS.H.; LeeJ.H.; YamadaM.; SethyR.; KawaiT.; JungJ.H. Angew. Chem., Int. Ed. 2019, 58, 18878. |
| [8] | (a) YangD.; DuanP.; ZhangL.; LiuM. Nat. Commun. 2017, 8, 15727. |
| [8] | (b) JiangH.; JiangY.; HanJ.; ZhangL.; LiuM. Angew. Chem., Int. Ed. 2019, 58, 785. |
| [8] | (c) HellouN.; Srebro-HooperM.; FavereauL.; ZinnaF.; CaytanE.; ToupetL.; DorcetV.; JeanM.; VanthuyneN.; WilliamsJ.A.G.; Di BariL.; AutschbachJ.; CrassousJ. Angew. Chem., Int. Ed. 2017, 56, 823. |
| [8] | (d) San Jose, B.A.; Matsushita, S.; Akagi, K. J. Am. Chem. Soc. 2012, 134, 19795. |
| [8] | (e) HanD.; HanJ.; HuoS.; QuZ.; JiaoT.; LiuM.; DuanP. Chem. Commun. 2018, 54, 5630. |
| [8] | (f) AokiR.; ToyodaR.; KögelJ.F.; SakamotoR.; KumarJ.; KitagawaY.; HaranoK.; Kawai T.; NishiharaH. J. Am. Chem. Soc. 2017, 139, 16024. |
| [8] | (g) ReinéP.; OrtuñoA.M.; ResaS.; Álvarez de CienfuegosL.; BlancoV.; Ruedas-RamaM.J.; MazzeoG.; AbbateS.; LucottiA.; TommasiniM.; Guisán-CeinosS.; RibagordaM.; CampañaA.G.; MotaA.; LonghiG.; MiguelD.; CuervaJ.M. Chem. Commun. 2018, 54, 13985. |
| [9] | (a) Jime?nezJ.-R.; DoistauB.; CruzC.M.; BesnardC.; CuervaJ.M.; Campan?aA.G.; PiguetC. J. Am. Chem. Soc. 2019, 141, 13244. |
| [9] | (b) AokiR.; ToyodaR.; Ko?gelJ.F.; SakamotoR.; KumarJ.; KitagawaY.; HaranoK.; KawaiT.; NishiharaH. J. Am. Chem. Soc. 2017, 139, 16024. |
| [9] | (c) HellouN.; Srebro-HooperM.; FavereauL.; ZinnaF.; CaytanE.; ToupetL.; DorcetV.; JeanM.; VanthuyneN.; WilliamsJ.A.G.; Di BariL.; AutschbachJ.; CrassousJ. Angew. Chem., Int. Ed. 2017, 56, 8236. |
| [10] | LunkleyJ.L.; ShirotaniD.; YamanariK.; KaizakiS.; MullerG. J. Am. Chem. Soc. 2008, 130, 13814. |
| [11] | YeungC.-T.; ChanW.T.K.; YanS.-C.; YuK.-L.; YimK.-H.; WongW.-T.; LawG.-L. Chem. Commun. 2015, 51, 592. |
| [12] | LiX.-Z.; ZhouL.-P.; YanL.-L.; YuanD.-Q.; LinC.-S.; SunQ.-F. J. Am. Chem. Soc. 2017, 139, 8237. |
| [13] | (a) StomeoF.; LincheneauC.; LeonardJ.P.; O'BrienJ.E.; PeacockR.D.; McCoyC.P.; GunnlaugssonT. J. Am. Chem. Soc. 2009, 131, 9636. |
| [13] | (b) BarryD.E.; KitchenJ.A.; PanduranganK.; SavyasachiA.J.; PeacockR.D.; GunnlaugssonT. Inorg. Chem. 2020, 59, 2646. |
| [13] | (c) CombyS.; StomeoF.; McCoyC.P.; GunnlaugssonT. Helv. Chim. Acta 2009, 92, 2461. |
| [13] | (d) KotovaO.; CombyS.; PanduranganK.; StomeoF.; O'BrienJ.E.; FeeneyM.; PeacockR.D.; McCoyC.P.; GunnlaugssonT. Dalton Trans. 2018, 47, 12308. |
| [13] | (e) LincheneauC.; PeacockR.D.; GunnlaugssonT. Chem. Asian J. 2010, 5, 500. |
| [14] | YeungC.-T.; YimK.-H.; WongH.-Y.; PalR.; LoW.-S.; YanS.-C.; WongM.Y.-M.; YufitD.; SmilesD.E.; McCormickL.J.; TeatS.J.; ShuhD.K.; WongW.-T.; LawG.-L. Nat. Commun. 2017, 8, 1128. |
| [15] | ZhangT.; ZhangG.-L.; ZhouL.-P.; GuoX.-Q.; SunQ.-F. Tetrahedron: Asymmetry 2017, 28, 550. |
| [16] | (a) ZhouY.; YaoY.; ChengZ.; GaoT.; LiH.; YanP. Inorg. Chem. 2020, 59, 12850. |
| [16] | (b) HanG.; ZhouY.; YaoY.; ChengZ.; GaoT.; LiH.; YanP. Dalton Trans. 2020, 49, 3312. |
| [17] | (a) ZhangJ.; ZhouY.; YaoY.; ChengZ.; GaoT.; LiH.; YanP. J. Mater. Chem. C 2020, 8, 6788. |
| [17] | (b) ChenA.Y.; ThomasP.W.; StewartA.C.; BergstromA.; ChengZ.; MillerC.; BethelC.R.; MarshallS.H.; CredilleC.V.; RileyC.L.; PageR.C.; BonomoR.A.; CrowderM.W.; TierneyD.L.; FastW.; CohenS.M. J. Med. Chem. 2017, 60, 7267. |
| [17] | (c) YanL.-L.; TanC.-H.; ZhangG.-L.; ZhouL.-P.; BünzliJ.-C.; SunQ.-F. J. Am. Chem. Soc. 2015, 137, 8550. |
| [17] | (d) LiX.-Z.; ZhouL.-P.; YanL.-L.; YuanD.-Q.; LinC.-S.; SunQ.-F. J. Am. Chem. Soc. 2017, 139, 8237. |
| [18] | DutraJ.D.L.; BispoT.D.; FreireR.O. J. Comput. Chem. 2014, 35, 772. |
| [19] | TelferS.G.; McLeanT.M.; WaterlandM.R. Dalton Trans. 2011, 40, 3097. |
| [20] | MullerG. InLuminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials, Ed.: de Bettencourt-Dias, A., Wiley, Hoboken, 2014, pp.77-124. |
| [21] | (a) BonsallS.D.; HoucheimeM.; StrausD.A.; MullerG. Chem. Commun. 2007, 35, 3676. |
| [21] | (b) PetoudS.; MullerG.; MooreE.G.; XuJ.; SokolnickiJ.; RiehlJ.P.; LeU.N.; CohenS.M.; RaymondK.N. J. Am. Chem. Soc. 2007, 129, 77. |
| [21] | (c) SeitzM.; DoK.; IngramA.J.; MooreE.G.; MullerG.; RaymondK.N. Inorg. Chem. 2009, 48, 8469. |
| [21] | (d) LeonardJ.P.; JensenP.; McCabeT.; O'BrienJ.E.; PeacockR.D.; KrugerP.E.; GunnlaugssonT. J. Am. Chem. Soc. 2007, 129, 10986. |
| [21] | (e) ShiN.; WangR.; WangX.; TanJ.; GuanY.; LiZ.; WanX.; ZhangJ. Chem. Commun. 2019, 55, 1136. |
| [21] | (f) YeungC.-T.; YimK.-H.; WongH.-Y.; PalR.; LoW.-S.; YanS.-C.; Yee-Man WongM.; YufitD.; SmilesD.E.; McCormickL.J.; TeatS.J.; ShuhD.K.; WongW.-T. LawG.-L. Nat. Commun. 2017, 8, 1128. |
| [21] | (g) GóreckiM.; CarpitaL.; ArricoL.; ZinnaF.; Di BariL. Dalton Trans. 2018, 47, 7166. |
| [21] | (h) KotovaO.; BlascoS.; TwamleyB.; O'BrienJ.; PeacockR.D.; KitchenJ.A.; Martínez-CalvoM.; GunnlaugssonT. Chem. Sci. 2015, 6, 457. |
| [21] | (i) OkutaniK.; NozakiK.; IwamuraM. Inorg. Chem. 2014, 53, 5527. |
| [21] | (j) LeonzioM.; MelchiorA.; FauraG.; TolazziM.; ZinnaF.; Di BariL.; PiccinelliF. Inorg. Chem. 2017, 56, 4413. |
| [22] | (a) HuaK.T.; XuJ.; QuirozE.E.; LopezS.; IngramA.J.; JohnsonV.A.; TischA.R.; de Bettencourt-DiasA.; StrausD.A.; MullerG. Inorg. Chem. 2012, 51, 647. |
| [22] | (b) LunkleyJ.L.; ShirotaniD.; YamanariK.; KaizakiS.; MullerG. Inorg. Chem. 2011, 50, 12724. |
| [23] | (a) MiyataK.; NakagawaT.; KawakamiR.; KitaY.; SugimotoK.; NakashimaT.; HaradaT.; Kawaiand T.; HasegawaY. Chem. - Eur. J. 2011, 17, 521. |
| [23] | (b) HasegawaY.; OhkuboT.; NakanishiT.; KobayashiA.; KatoM.; SekiT.; ItoH.; FushimiK. Eur. J. Inorg. Chem. 2013, 5911. |
| [23] | (c) ZhuT.; ChenP.; LiH.; SunW.; GaoT.; YanP. Phys. Chem. Chem. Phys. 2015, 17, 16136. |
| [24] | (a) LatvaM.; TakaloH.; MukkalaV.-M.; MatachescuC.; Rodriguez-UbisJ.C.; KankareJ. J. Lumin. 1997, 75, 149. |
| [24] | (b) SteemersF.J.; VerboomW.; ReinhoudtD.N.; van der TolE.B.; VerhoevenJ.W. J. Am. Chem. Soc. 1995, 117, 9408. |
/
| 〈 |
|
〉 |