

双齿氮配体螯合五价碘试剂介导的苯酚氧化去芳构化机理的理论研究
收稿日期: 2021-08-01
网络出版日期: 2021-09-10
基金资助
项目受国家自然科学基金(21933004); 项目受国家自然科学基金(21772098); 中央高校基本科研业务费专项资金资助
Mechanistic Study on the Bidentate Nitrogen-Ligated Iodine(V) Reagent Promoted Oxidative Dearomatization of Phenols
Received date: 2021-08-01
Online published: 2021-09-10
Supported by
National Natural Science Foundation of China(21933004); National Natural Science Foundation of China(21772098); Fundamental Research Funds for the Central Universities
张丹琪 , 邵英博 , 郑汉良 , 周碧莹 , 薛小松 . 双齿氮配体螯合五价碘试剂介导的苯酚氧化去芳构化机理的理论研究[J]. 化学学报, 2021 , 79(11) : 1394 -1400 . DOI: 10.6023/A21080358
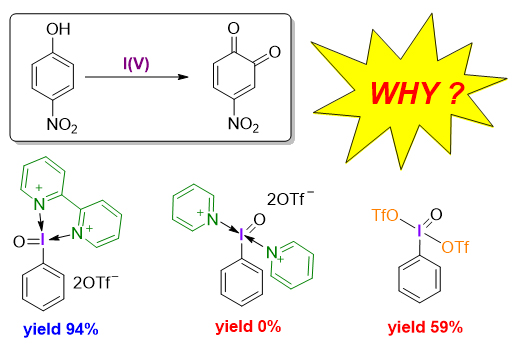
Hypervalent iodine reagents, as a type of environmentally friendly and economical oxidants, have received extensive attention from synthetic chemistry in recent years. There are two kinds of common hypervalent iodine reagents: iodine(III) and iodine(V) reagents. Among various iodine(V) based reagents, 2-iodoxybenzoic acid (IBX) is one of the most commonly used oxidants in organic chemistry and has been widely used in selective oxidation of phenols, alcohols, thioethers and many other compounds. Although the IBX can efficiently oxidize electron-rich phenols to obtain o-quinones, which have important applications in catalysis and materials science, the synthesis of electron-deficient o-quinones is still challenging and remains an unsolved problem. Recently, a novel bidentate nitrogen-ligated iodine(V) reagent has been found to efficiently and selectively promote the oxidative dearomatization of electron-deficient phenols to o-quinones. The bidentate-nitrogen ligand has been demonstrated to play a crucial role in the successful oxidative dearomatization of electron-deficient phenols. To understand the origin of the effect of bidentate-nitrogen ligand on oxidative dearomatization, we herein conducted a detailed mechanistic study on the oxidative dearomatization of phenols promoted by iodine(V) reagents with different ligands by using density functional theory (DFT). Geometry optimizations and frequency calculations were carried out at the M06-2X/[6-31G(d,p)+LANL2DZ(I)] level of theory. To obtain more accurate electronic energies, single-point energy were calculated at the M06-2X/[6-311+G(2d,p)+def2-TZVPP(I)] level of theory. The solvation model based on density (SMD) was used to account for the solvation effect of chloroform, the solvents used in the experiment. Calculations revealed that the bidentate-nitrogen ligand not only enhances the reactivity of iodine(V) reagent but also acts as a base to neutralize the strong acid generated in the reaction to prevent product degradation. The insights into the effect of the bidentate-nitrogen ligand on the reactivity of hypervalent iodine reagent obtained from this study would facilitate the future design of novel ligand- regulated hypervalent iodine reagents for new reactions.
| [1] | (a) Zhdankin V. V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, New York, 2013. |
| [1] | (b) Chen J.; Qu H.; Peng J.; Chen C. Chin. J. Org. Chem. 2015, 35, 937. (in Chinese) |
| [1] | ( 陈静, 曲红梅, 彭静, 陈超, 有机化学, 2015, 35, 937.) |
| [1] | (c) Duan Y.; Jiang S.; Han Y.; Sun B.; Zhang C. Chin. J. Org. Chem. 2016, 36, 1973. (in Chinese) |
| [1] | ( 段亚南, 姜山, 韩永超, 孙博, 张弛, 有机化学, 2016, 36, 1973.) |
| [1] | (d) Yoshimura A.; Zhdankin V. V. Chem. Rev. 2016, 116, 3328. |
| [1] | (e) Zhang X.; Cong Y.; Lin G.; Guo X.; Cao Y.; Lei K.; Du Y. Chin. J. Org. Chem. 2016, 36, 2513. (in Chinese) |
| [1] | ( 张翔, 丛颖, 林光宇, 郭旭亮, 曹阳, 雷坤华, 杜云飞, 有机化学, 2016, 36, 2513.) |
| [1] | (f) Ma J.; Chen L.; Yuan Z.; Cheng H. Chin. J. Org. Chem. 2018, 38, 1586. (in Chinese) |
| [1] | ( 马姣丽, 陈立成, 袁中文, 程辉成, 有机化学, 2018, 38, 1586.) |
| [1] | (g) Parra A. Chem. Rev. 2019, 119, 12033. |
| [1] | (h) Cai Q.; Ma H. Acta Chim. Sinica 2019, 77, 213. (in Chinese) |
| [1] | ( 蔡倩, 马浩文, 化学学报, 2019, 77, 213.) |
| [1] | (i) Zhang B.; Li X.; Guo B.; Du Y. Chem. Commun. 2020, 56, 14119. |
| [1] | (j) Yang X.; Hu Z.; Jia M.; Du F.; Zhang C. Synlett 2021, 32, 1289. |
| [2] | (a) Qin K.; Su G.; Rao W.; Tan G. M. Chin. J. Org. Chem. 2006, 26, 1623. (in Chinese) |
| [2] | ( 覃开云, 苏桂发, 饶万平, 谭光明, 有机化学, 2006, 26, 1623.) |
| [2] | (b) Satam V.; Harad A.; Rajule R.; Pati H. Tetrahedron 2010, 66, 7659. |
| [2] | (c) Duschek A.; Kirsch S. F. Angew. Chem. Int. Ed. 2011, 50, 1524. |
| [2] | (d) Zhang S.; Wu H.; Tang Y. Chin. J. Org. Chem. 2021, 41, 490. (in Chinese) |
| [2] | ( 张书瑜, 吴昊天, 汤峨, 有机化学, 2021, 41, 490.) |
| [3] | (a) Pierpont C. G. Coord. Chem. Rev. 2001, 216, 99. |
| [3] | (b) Kharisov B. I.; Méndez-Rojas M. A.; Garnovskii A. D.; Ivakhnenko E. P.; Ortiz-Méndez U. J. Coord. Chem. 2002, 55, 745. |
| [3] | (c) Sun L.; Campbell M. G.; Dinca M. Angew. Chem. Int. Ed. 2016, 55, 3566. |
| [3] | (d) Sato S.; Sakata K.; Hashimoto Y.; Takikawa H.; Suzuki K. Angew. Chem. Int. Ed. 2017, 56, 12608. |
| [3] | (e) Esguerra K. V. N.; Lumb J.-P. Angew. Chem. Int. Ed. 2018, 57, 1514. |
| [3] | (f) Zhang R.; Luo S. Chin. Chem. Lett. 2018, 29, 1193. |
| [4] | (a) Magdziak D.; Rodriguez A. A.; Van De Water R. W.; Pettus T. R. Org. Lett. 2002, 4, 285. |
| [4] | (b) Lebrasseur N.; Gagnepain J.; Ozanne-Beaudenon A.; Léger J. M.; Quideau S. J. Org. Chem. 2007, 72, 6280. |
| [4] | (c) Wu A.; Duan Y.; Xu D.; Penning T. M.; Harvey R. G. Tetrahedron 2010, 66, 2111. |
| [4] | (d) Uyanik M., Mutsuga T., Ishihara K. Molecules 2012, 17, 8604. |
| [4] | (e) Usui K.; Yamamoto K.; Shimizu T.; Okazumi M.; Mei B.; Demizu Y.; Kurihara M.; Suemune H. J. Org. Chem. 2015, 80, 6502. |
| [4] | (f) Mishra A. K.; Moorthy J. N. J. Org. Chem. 2016, 81, 6472. |
| [4] | (g) Uyanik M.; Mutsuga T.; Ishihara K. Angew. Chem. Int. Ed. 2017, 56, 3956. |
| [4] | (h) Pulvirenti L.; Muccilli V.; Cardullo N.; Spatafora C.; Tringali C. J. Nat. Prod. 2017, 80, 1648. |
| [4] | (i) Bizzarri B. M.; Botta L.; Capecchi E.; Celestino I.; Checconi P.; Palamara A. T.; Nencioni L.; Saladino R. J. Nat. Prod. 2017, 80, 3247. |
| [4] | (j) Stack D. E.; Mahmud B. Synth. Commun. 2018, 48, 161. |
| [5] | (a) Xiao X.; Greenwood N. S.; Wengryniuk S. E. Angew. Chem., Int. Ed. 2019, 131, 16327. |
| [5] | (b) Xiao X.; Roth J. M.; Greenwood N. S.; Velopolcek M. K.; Aguirre J.; Jalali M.; Ariafard A.; Wengryniuk S. E. J. Org. Chem. 2021, 86, 6566. |
| [6] | (a) Weiss R.; Seubert J. Angew. Chem. Int. Ed. 1994, 33, 891. |
| [6] | (b) Zhdankin V. V.; Koposov A. Y.; Yashin N. V. Tetrahedron Lett. 2002, 43, 5735. |
| [7] | (a) Zhou B.; Yan T.; Xue, X. S.; Cheng, J. P. Org. Lett. 2016, 18, 6128. |
| [7] | (b) Yan T.; Zhou, B.; Xue, X. S.; Cheng, J. P. J. Org. Chem. 2016, 81, 9006. |
| [7] | (c) Zhou B.; Xue X. S.; Cheng J. P. Tetrahedron Lett. 2017, 58, 1287. |
| [7] | (d) Zhou B.; Haj M. K.; Jacobsen E. N.; Houk K. N.; Xue X. S. J. Am. Chem. Soc. 2018, 140, 15206. |
| [7] | (e) Zheng H.; Sang Y.; Houk K. N.; Xue X. S.; Cheng J. P. J. Am. Chem. Soc. 2019, 141, 16046. |
| [7] | (f) Yang J.; Li M.; Xue X. S. Chin. J. Chem. 2019, 37, 359. |
| [7] | (g) Zheng H.; Xue X. S. Curr. Org. Chem. 2020, 4, 1. |
| [8] | Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F. Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery Jr., J. A.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016. |
| [9] | Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 6378. |
| [10] | Zhao Y.; Truhlar D. G. Acc. Chem. Res. 2008, 41, 157. |
| [11] | Hay P. J.; Wadt W. R. J. Chem. Phys. 1985, 82, 299. |
| [12] | (a) Weigend F.; Furche F.; Ahlrichs R. J. Chem. Phys. 2003, 119, 12753. |
| [12] | (b) Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297. |
| [12] | (c) Jiang H.; Sun T. Y.; Wang X.; Xie Y.; Zhang X.; Wu Y. D.; Schaefer III H. F. Org. Lett. 2017, 19, 6502. |
| [12] | (d) Sun T.; Chen K.; Zhou H.; You T.; Yin P.; Wang X. J. Comput. Chem. 2021, 42, 470. |
| [13] | Legault C. Y. CYLview, 1.0b, Université de Sherbrooke, 2009; |
| [14] | The PyMOL Molecular Graphics System, Version 2.0.4, Schrödinger, LLC. |
| [15] | Kaur A.; Ariafard A. Org. Biomol. Chem. 2020, 18, 1117. |
| [16] | (a) Feldman K. S.; Sambandam A.; Bowers K. E.; Appel H. M. J. Org. Chem. 1999, 64, 5794. |
| [16] | (b) Mitchell J. S.; Wu Y.; Cook C. J.; Main L. Bioconjugate Chem. 2007, 18, 268. |
| [17] | 在审稿期间,Ariafard等人在有机化学杂志发表了相关反应的机理研究工作, 该工作得到了与我们工作相似的结论: Jalali M.; Bissember A. C.; Yates B. F..; Wengryniuk S. E.; Ariafard A. J. Org. Chem. 2021, 86, 12237. |
/
| 〈 |
|
〉 |