

一种新型磺酸修饰的共价有机框架用于二氧化碳吸收和染料吸附
收稿日期: 2021-10-11
网络出版日期: 2021-11-23
基金资助
项目受国家自然科学基金(22025504)
A New Covalent Organic Framework Modified with Sulfonic Acid for CO2 Uptake and Selective Dye Adsorption
Received date: 2021-10-11
Online published: 2021-11-23
Supported by
National Natural Science Foundation of China(22025504)
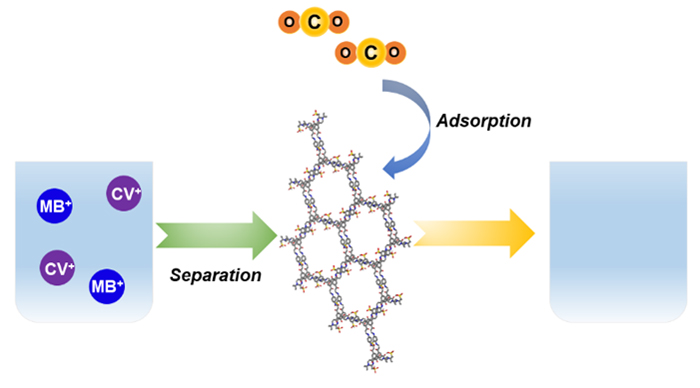
环境污染是地球现今的重要问题之一, 而其中就包括水污染与温室效应. 共价有机框架(covalent organic frameworks, COFs)作为一种新兴的晶态多孔聚合物, 因其优异的吸附性能, 在污染治理领域具有广阔的应用前景. 本工作报道了一种基于β-酮胺单体通过迈克尔加成-消除反应合成的磺酸型微孔COF (JUC-603). 该COF不仅能有效分离水中染料, 而且具有显著的二氧化碳吸收能力. 本研究为环境修复中的染料分离和二氧化碳吸收领域提供了一种具有潜力的材料.
关键词: 多孔材料; 共价有机框架; 染料吸附; 二氧化碳吸收; 迈克尔加成-消除反应
王自陶 , 刘耀祖 , 王钰杰 , 方千荣 . 一种新型磺酸修饰的共价有机框架用于二氧化碳吸收和染料吸附[J]. 化学学报, 2022 , 80(1) : 37 -43 . DOI: 10.6023/A21100454
Environmental pollution is one of the most severe problems facing the earth today, including water pollution and the greenhouse effect caused by the indiscriminate discharge of organic dyes and abundant carbon dioxide emissions (CO2), respectively. Therefore, removing dyes from water and eliminating CO2 in the atmosphere is an urgent issue that needs to be solved. Among them, with low cost and high adsorption efficiency, porous materials have become one of the most common materials to adsorb organic dyes and uptake CO2. Covalent organic frameworks (COFs), as a burgeoning class of crystalline porous polymers, present a promising application potential in many areas, such as gas adsorption and separation, heterogeneous catalysis, semiconductors and sensors due to their regular pores, modifiable frameworks, extensive specific areas and excellent stability. Especially the introduction of the functional groups to the skeleton of COFs provides abundant active sites to adsorb specific dyes and uptake gases. Inspired by this, we report a microporous COF (JUC-603, JUC=Jilin University China) decorated with sulfonic acid group. This COF was successfully synthesized through β-ketoenamine based Michael addition-elimination reaction of 1,3,5-tris(3-dimethylamino-1-oxoprop-2-en-yl)benzene (TDOEB) and p-phenylenediamine (PPDA). A series of characterizations proved that JUC-603 had high crystallinity, opening microporous pore and excellent stability. Nitrogen adsorption-desorption isotherm revealed a high Brunauer-Emmett-Teller (BET) surface area (774 m2/g) and large pore size (1.6 nm). Moreover, we studied the adsorption of JUC-603 on dyes from aqueous solutions by ultraviolet- visible spectrophotometry and the uptake capacity through CO2 adsorption-desorption. These results showed that JUC-603 could selectively adsorb cationic dyes such as crystal violet and methylene blue as well as uptake CO2 effectively (52 cm3/g at 273 K and 0.1 MPa). The ideal performance was attributed to the modulation of active sulfonic acid sites on the framework of JUC-603. This research thus indicates that the COF as a promising functionalized porous material has great potential in environmental remediation.

| [1] | Nugent, P.; Belmabkhout, Y.; Burd, S. D.; Cairns, A. J.; Luebke, R.; Forrest, F.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; Eddaoudi, M.; Zaworotko, M. J. Nature 2013, 495, 80. |
| [2] | Pachauri, R. K.; Reisinger, A. IPCC Fifth Assessment Report, 2014. |
| [3] | Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O′Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Science 2005, 310, 1166. |
| [4] | Colson, J. W.; Woll, A. R.; Mukherjee, A.; Levendorf, M. P.; Spitler, E. L.; Shields, V. B.; Spencer, M. G.; Park, J.; Dichtel, W. R. Science 2011, 332, 228. |
| [5] | Feng, X.; Ding, X. S.; Jiang, D. L. Chem. Soc. Rev. 2012, 41, 6010. |
| [6] | Ding, S.; Wang, W. Chem. Soc. Rev. 2013, 42, 548. |
| [7] | Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Chem. Soc. Rev. 2020, 49, 1357. |
| [8] | Kuhn, P.; Antonietti, M.; Thomas, A. Angew. Chem. Int. Ed. 2008, 47, 3450. |
| [9] | Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; Wang, B. J. Am. Chem. Soc. 2017, 139, 4258. |
| [10] | Wang, X.; Han, X.; Zhang, J.; Wu, X.; Liu, Y.; Cui, Y. J. Am. Chem. Soc. 2016, 138, 12332. |
| [11] | Sun, Q.; Aguila, B.; Perman, J.; Nguyen, N.; Ma, S. Q. J. Am. Chem. Soc. 2016, 138, 15790. |
| [12] | Chandra, S.; Kandambeth, T. S.; BabaRao, R.; Marathe, M. Y.; Kunjir, S. M.; Banerjee, R. J. Am. Chem. Soc. 2014, 136, 6570. |
| [13] | Liang, R.; Cui, F.; Ru-Han, A.; Qi, Q.; Zhao, X. CCS Chem. 2020, 2, 139. |
| [14] | Wang, H.; Zeng, Z.; Xu, P.; Li, L.; Zeng, G.; Xiao, R.; Tang, Z.; Huang, D.; Tang, L.; Lai, C.; Jiang, D.; Liu, Y.; Yi, H.; Qin, L.; Ye, S.; Ren, X.; Tang, W. Chem. Soc. Rev. 2019, 48, 488. |
| [15] | Wang, Z.; Li, H.; Yan, S.; Fang, Q. Acta Chim. Sinica 2020, 78, 63 ; (in Chinese) |
| [15] | ( 王志涛, 李辉, 颜士臣, 方千荣, 化学学报 2020, 78, 63.) |
| [16] | Chang, J.; Xu, G.; Li, H.; Fang, Q. Chem. J. Chinese Univ. 2020, 41, 1609 ; (in Chinese) |
| [16] | ( 常建红, 徐国杰, 李辉, 方千荣, 高等学校化学学报, 2020, 41, 1609.) |
| [17] | Fang, J.; Zhao, W.; Zhang, M.; Fang, Q. Acta Chim. Sinica 2021, 79 186 ; (in Chinese) |
| [17] | ( 方婧, 赵文娟, 张明浩, 方千荣, 化学学报 2021, 79, 186.) |
| [18] | Liu, J.; Zhang, M.; Wang, N.; Wang, C.; Ma, L. Acta Chim. Sinica 2020, 78, 311 ; (in Chinese) |
| [18] | ( 刘建国, 张明月, 王楠, 王晨光, 马隆龙, 化学学报 2020, 78, 311.) |
| [19] | Peng, Z.; Ding, H.; Chen, R.; Gao, C.; Wang, C. Acta Chim. Sinica 2019, 77, 681 ; (in Chinese) |
| [19] | ( 彭正康, 丁慧敏, 陈如凡, 高超, 汪成, 化学学报 2019, 77, 681.) |
| [20] | Wang, T.; Zhao, L.; Wang, K.; Bai, Y.; Feng, F. Acta Chim. Sinica 2021, 79, 600 ; (in Chinese) |
| [20] | ( 王涛, 赵璐, 王科伟, 白云峰, 冯峰, 化学学报 2021, 79, 600.) |
| [21] | Luo, Y.; Li, B.; Wang, W.; Wu, K.; Tan, B. Adv. Mater. 2021, 24, 5703. |
| [22] | Demessence, A.; Alessandro, D. M.; Foo, L.; Long, J. R. J. Am. Chem. Soc. 2009, 131, 8784. |
| [23] | Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H. J. Am. Chem. Soc. 2011, 133, 18126. |
| [24] | Rao, M. R.; Fang, Y.; Feyter, S. D.; Perepichka, D. F. J. Am. Chem. Soc. 2017, 139, 2421. |
| [25] | Liu, Y.; Wang, Y.; Li, H.; Guan, X.; Zhu, L.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Chem. Sci. 2019, 10, 10815. |
| [26] | Materials Studio, Ver. 7.0, Accelrys Inc., San Diego, CA. |
/
| 〈 |
|
〉 |