

可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应
收稿日期: 2021-10-13
网络出版日期: 2021-12-06
基金资助
项目受国家自然科学基金(21871053); 项目受国家自然科学基金(22007017)
Visible-light Photocatalytic Alkylsulfonylation of Aroylhydrazides with Alkylsulfonyl Radicals
Received date: 2021-10-13
Online published: 2021-12-06
Supported by
National Natural Science Foundation of China(21871053); National Natural Science Foundation of China(22007017)
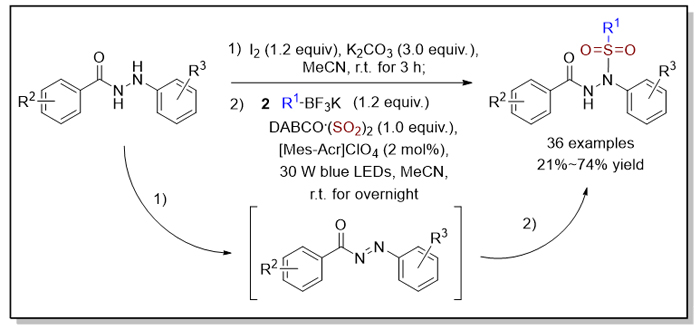
鉴于磺酰基在有机分子中的重要意义, 磺酰基的引入与磺酰化合物的合成被广泛报道. 其中, 磺酰肼化合物因在抗肿瘤、抗菌等方面表现出的生物活性, 其相关合成备受关注. 本工作发展了可见光照射下烷基三氟硼酸钾、DABCO•(SO2)2 (1,4-Diazabicyclo[2.2.2]octane, DABCO)和芳酰肼的有机光反应, 一锅法获得了中等到良好收率的N'-酰基-N-磺酰肼化合物, 该反应底物适用性广、绿色温和. 机理研究表明该反应经历自由基反应历程, 即由烷基三氟硼酸钾在光催化作用下原位生成的烷基自由基引发的, 随后通过二氧化硫插入形成烷基磺酰基自由基中间体, 最后被芳酰肼氧化产生的N-酰基二氮烯中间体捕获, 从而生成N'-酰基-N-磺酰化产物.
杨民 , 叶柏柏 , 陈健强 , 吴劼 . 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022 , 80(1) : 11 -15 . DOI: 10.6023/A21100457
In view of the importance of sulfonyl groups in organic molecules, the introduction of sulfonyl groups and the synthesis of sulfonyl compounds have been widely reported. Among them, the synthesis of sulfonylhydrazides has attracted much attention because of their biological activities in antitumor and antibacterial activities. Here, we developed a photoinduced reaction of potassium alkyltrifluoroborates, DABCO•(SO2)2 (1,4-Diazabicyclo[2.2.2]octane, DABCO), and aroylhydrazides under visible light irradiation, which obtained N'-acyl-N-alkylsulfonylhydrazides in moderate to good yields in one-pot. This reaction works in a green and mild way with a broad substrate scope. Mechanistic study shows that after giving rise to N-acyldiazenes via the oxidation of aroylhydrazides, the transformation is initiated by alkyl radicals generated in situ from potassium alkyltrifluoroborates in the presence of photocatalyst. The subsequent insertion of sulfur dioxide and alkylsulfonylation of N-acyldiazenes with alkylsulfonyl radical intermediates afford the corresponding N'-acyl-N-alkylsulfonyl- hydrazides. General procedure for visible-light photocatalytic alkylsulfonylation of aroylhydrazides with alkylsulfonyl radicals: to a solution of aroylhydrazides 1 (0.2 mmol) and K2CO3 (0.3 mmol) in MeCN (2.0 mL) was added I2 (0.12 mmol). The reaction was stirred for 3 h at room temperature, then quenched with sat. aq. Na2S2O3 (15 mL). The layers were separated and the aqueous layer extracted with dichloromethane (DCM) (15 mL×3). The combined organic layers were then washed with brine (15 mL), and dried over Na2SO4. Evaporation of the solvent under reduced pressure afforded the crude N-acyldiazenes, which was used in the subsequent transformation without further purification. The crude N-acyldiazenes were combined with potassium alkyltrifluoroborates 2 (0.24 mmol), DABCO•(SO2)2 (0.2 mmol) and 9-mesityl-10-methylacridinium perchlorate ([Mes-Acr]ClO4) (0.004 mmol) in a tube. The tube was evacuated and backfilled with N2 three times before the addition of MeCN (2.0 mL). The mixture was then placed around blue light emitting diodes (LEDs) (30 W) with a distance of 10 cm, and was stirred under blue light irradiation for overnight at room temperature. After completion of reaction as indicated by thin layer chromatography (TLC), the solvent was evaporated and the residue was purified directly by flash column chromatography (V(n-hexane)/V(ethyl acetate)=5∶1—2∶1) to give the N'-acyl-N-sulfonylhydrazides 3.

| [1] | (a) Prinsep, M. R.; Blunt, J. W.; Munro, M. H. G. J. Nat. Prod. 1991, 54, 1068. |
| [1] | (b) Teall, M.; Oakley, P.; Harrison, T. Bioorg. Med. Chem. Lett. 2005, 15, 2685. |
| [1] | (c) Legros, L.; Dehli, J. R.; Bolm, C. Adv. Synth. Catal. 2005, 347, 19. |
| [1] | (d) Churcher, I.; Beher, D.; Best, J. D. Bioorg. Med. Chem. Lett. 2006, 16, 280. |
| [1] | (e) Harrak, Y.; Casula, G.; Basset, J. J. Med. Chem. 2010, 53, 6560. |
| [2] | Simpkins, N. S. Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993. |
| [3] | (a) Hayakawa, M.; Kaizawa, H.; Kawaguchi, K. I.; Ishikawa, N.; Koizumi, T.; Ohishi, T.; Workman, P. Bioorg. Med. Chem. 2007, 15, 403. |
| [3] | (b) Kamal, A.; Khan, M. N. A.; Reddy, K. S.; Rohini, K. Bioorg. Med. Chem. 2007, 15, 1004. |
| [3] | (c) Kendall, J. D.; Rewcastle, G. W.; Frederick, R.; Mawson, C.; Denny, W. A.; Marshall, E. S.; Shepherd, P. R. Bioorg. Med. Chem. 2007, 15, 7677. |
| [3] | (d) Riaz, S.; Khan, I. U.; Bajda, M.; Ashraf, M.; Shaukat, A.; Rehman, T. U.; Yar, M. Bioorg. Chem. 2015, 63, 64. |
| [3] | (e) Wu, G.; Chen, R.; Yang, K.; Liu, H.; Wang, C. Chem. Res. Appl. 2020, 32, 1053. (in Chinese) |
| [3] | ( 吴桂贞, 陈任宏, 杨凯, 刘海, 汪朝阳, 化学研究与应用, 2020, 32, 1053) |
| [4] | (a) Zheng, D.; An, Y.; Li, Z.; Wu, J. Angew. Chem. Int. Ed. 2014, 53, 2451. |
| [4] | (b) Namba, K.; Takeuchi, K.; Kaihara, Y.; Oda, M.; Nakayama, A.; Nakayama, A.; Tanino, K. Nat. Commun. 2015, 6, 1. |
| [4] | (c) Huang, Z.; Wang, C.; Dong, G. Angew. Chem. Int. Ed. 2016, 55, 5299. |
| [4] | (d) Sun, Q.; Li, L.; Liu, L.; Guan, Q.; Yang, Y.; Zha, Z.; Wang, Z. Org. Lett. 2018, 20, 5592. |
| [5] | (a) Wang, Y.; Wang, H.; Liu, Z. Acta Chim. Sinica 2021, 79, 1085. (in Chinese) |
| [5] | ( 王也铭, 王宏伟, 刘兆洪, 化学学报 2021, 79, 1085.) |
| [5] | (b) Ferwanah, A.-R.; Awadallah, A. Molecules 2005, 10, 492. |
| [5] | (c) Sun, J.; Qiu, J.-K.; Zhu, Y.-L.; Guo, C.; Hao, W.-J.; Jiang, B.; Tu, S.-J. J. Org. Chem. 2015, 80, 8217. |
| [5] | (d) Sun, J.; Qiu, J.-K.; Jiang, B.; Hao, W.-J.; Guo, C.; Tu, S.-J. J. Org. Chem. 2016, 81, 3321. |
| [5] | (e) Sun, K.; Wang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490. |
| [5] | (f) Wan, X.; Sun, K.; Zhang, G. Sci. China: Chem. 2017, 60, 353. |
| [5] | (g) Sun, K.; Lv, Y.; Shi, Z.; Fu, F.; Zhang, C.; Zhang, Z. Sci. China: Chem. 2017, 60, 730. |
| [5] | (h) Yang, Y.; Bao, Y. J.; Guan, Q. Q.; Sun, Q.; Zha, Z. G.; Wang, Z. Y. Green Chem. 2017, 19, 112. |
| [6] | (a) Merino, E. Chem. Soc. Rev. 2011, 40, 3835. |
| [6] | (b) Rosenbaum, C.; Waldmann, H. Tetrahedron Lett. 2001, 42, 5677. |
| [6] | (c) White, E. H.; Field, K. W.; Hendrickson, W. H.; Dzadzic, P.; Roswell, D. F.; Paik, S.; Mullen, P. W. J. Am. Chem. Soc. 1992, 114, 8023. |
| [6] | (d) Millington, C. R.; Quarrell, R.; Lowe, G. Tetrahedron Lett. 1998, 39, 7201. |
| [7] | (a) Bandara, H. M. D.; Burdette, S. C. Chem. Soc. Rev. 2012, 41, 1809. |
| [7] | (b) Tsupova, S.; Meorg, U. Heterocycles 2014, 88, 129. |
| [8] | (a) Waring, D. R.; Hallas, G. The chemistry and application of dyes, Plenum, New York, 1990. |
| [8] | (b) Hunger, K. Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH, Weinheim, 2003. |
| [9] | (a) Yoneda, F.; Suzuki, K.; Nitta, Y. J. Am. Chem. Soc. 1966, 88, 2328. |
| [9] | (b) Yoneda, F.; Suzuki, K.; Nitta, Y. J. Org. Chem. 1967, 32, 727. |
| [9] | (c) Cao, H. T.; Grée, R. Tetrahedron Lett. 2009, 50, 1493. |
| [9] | (d) Stone, M. T. Org. Lett. 2011, 13, 2326. |
| [9] | (e) Bøgevig, A.; Juhl, K.; Kumaragurubaran, N.; Zhuang, W.; Jørgensen, K. A. Angew. Chem. Int. Ed. 2002, 41, 1790. |
| [9] | (f) Liu, T.-Y.; Cui, H.-L.; Zhang, Y.; Jiang, K.; Du, W.; He, Z.-Q.; Chen, Y.-C. Org. Lett. 2007, 9, 3671. |
| [9] | (g) Cheng, L.; Liu, L.; Wang, D.; Chen, Y.-J. Org. Lett. 2009, 11, 3874. |
| [9] | (h) Lan, Q.; Wang, X.; He, R.; Ding, C.; Maruoka, K. Tetrahedron Lett. 2009, 50, 3280. |
| [9] | (i) Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300. |
| [9] | (j) Sharma, S.; Han, S. H.; Han, S.; Ji, W.; Oh, J.; Lee, S.-Y.; Oh, J. S.; Jung, Y. H.; Kim, I. S. Org. Lett. 2015, 17, 2852. |
| [10] | (a) Forchiassin, M.; Risaliti, A.; Russo, C. Tetrahedron 1981, 37, 2921. |
| [10] | (b) Chan, A.; Scheidt, K. A. J. Am. Chem. Soc. 2008, 130, 2740. |
| [10] | (c) Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. Angew. Chem. Int. Ed. 2009, 48, 192. |
| [10] | (d) Yang, L.; Wang, F.; Lee, R.; Lv, Y.; Huang, K.-W.; Zhong, G. Org. Lett. 2014, 16, 3872. |
| [10] | (e) Morrill, L. C.; Lebl, T.; Slawin, A. M. Z.; Smith, A. D. Chem. Sci. 2012, 3, 2088. |
| [10] | (f) Zhang, Q.; Meng, L.-G.; Zhang, J.; Wang, L. Org. Lett. 2015, 17, 3272. |
| [10] | (g) Savva, A. C.; Mirallai, S. I.; Zissimou, G. A.; Berezin, A. A.; Demetriades, M.; Kourtellaris, A.; Constantinides, C. P.; Nicolaides, C.; Trypiniotis, T.; Koutentis, P. A. J. Org. Chem. 2017, 82, 7564. |
| [10] | (h) Zhou, R.; Han, L.; Zhang, H.; Liu, R.; Li, R. Adv. Synth. Catal. 2017, 359, 3977. |
| [10] | (i) Ma, C.; Zhou, J.-Y.; Zhang, Y.-Z.; Mei, G.-J.; Shi, F. Angew. Chem. Int. Ed. 2018, 57, 5398. |
| [11] | (a) Bisseret, P.; Blanchard, N. Org. Biomol. Chem. 2013, 11, 5393. |
| [11] | (b) Deeming, A. S.; Emmett, E. J.; Richards-Taylor, C. S.; Willis, M. C. Synthesis, 2014, 46, 2701. |
| [11] | (c) Liu, G.; Fan, C.; Wu, J. Org. Biomol. Chem. 2015, 13, 1592. |
| [11] | (d) Emmett, E. J.; Willis, M. C. Asian J. Org. Chem. 2015, 4, 602. |
| [11] | (e) Zheng, D.; Wu, J. Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017. |
| [11] | (f) Qiu, G.; Zhou, K.; Gao, L.; Wu, J. Org. Chem. Front. 2018, 5, 691. |
| [11] | (g) Hofman, K.; Liu, N. W.; Manolikakes, G. Chem. Eur. J. 2018, 24, 11852. |
| [11] | (h) Qiu, G.; Lai, L.; Cheng, J.; Wu, J. Chem. Commun. 2018, 54, 10405. |
| [11] | (i) Qiu, G.; Zhou, K.; Wu, J. Chem. Commun. 2018, 54, 12561. |
| [11] | (j) Ye, S.; Qiu, G.; Wu, J. Chem. Commun. 2019, 55, 1013. |
| [11] | (k) Ye, S.; Yang, M.; Wu, J. Chem. Commun. 2020, 56, 4145. |
| [11] | (l) Ye, S.; Li, X.; Xie, W.; Wu, J. Eur. J. Org. Chem. 2020, 2020, 1274. |
| [11] | (m) Xiao, W.; Wang, X.; Liu, R.; Wu, J. Chin. Chem. Lett. 2021, 32, 1847. |
| [12] | (a) Wang, X.; Xue, L.; Wang, Z. Org. Lett. 2014, 16, 4056. |
| [12] | (b) Liu, N. W.; Liang, S.; Manolikakes, G. Adv. Synth. Catal. 2017, 359, 1308. |
| [12] | (c) Wang, H.; Sun, S.; Cheng, J. Org. Lett. 2017, 19, 5844. |
| [12] | (d) Tang, N.; Shao, X.; Wang, M.; Wu, X.; Zhu, C. Acta Chim. Sinica 2019, 77, 922. (in Chinese) |
| [12] | ( 汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨, 化学学报 2019, 77, 922) |
| [12] | (e) Sheng, J.; Li, Y.; Qiu, G. Org. Chem. Front. 2017, 4, 95. |
| [12] | (f) Wang, Y.; Deng, L.; Zhou, J. Adv. Synth. Catal. 2018, 360, 1060. |
| [12] | (g) Tribby, A. L.; Rodríguez, I.; Shariffudin, S.; Ball, N. D. J. Org. Chem. 2017, 82, 2294. |
| [13] | (a) Zheng, D.; Yu, J.; Wu, J. Angew. Chem. Int. Ed. 2016, 55, 11925. |
| [13] | (b) Zhang, J.; An, Y.; Wu, J. Chem. Eur. J. 2017, 23, 9477. |
| [13] | (c) Liu, T.; Zheng, D.; Li, Z.; Wu, J. Adv. Synth. Catal. 2017, 359, 2653. |
| [13] | (d) An, Y.; Wu, J. Org. Lett. 2017, 19, 6028. |
| [13] | (e) Liu, T.; Zheng, D.; Ding, Y.; Fan, X.; Wu, J. Chem. Asian J. 2017, 12, 465. |
| [13] | (f) An, Y.; Zhang, J.; Xia, H.; Wu, J. Org. Chem. Front. 2017, 4, 1318. |
| [13] | (g) Wang, X.; Liu, T.; Zhong, Q.; Wu, J. Org. Chem. Front. 2017, 4, 2455. |
| [13] | (h) Liu, T.; Zheng, D.; Wu, J. Org. Chem. Front. 2017, 4, 1079. |
| [13] | (i) Zhang, J.; Zhou, K.; Wu, J. Org. Chem. Front. 2018, 5, 813. |
| [13] | (j) Liu, T.; Zheng, D.; Li, Z.; Wu, J. Adv. Synth. Catal. 2018, 360, 865. |
| [13] | (k) Zhang, J.; Zhang, F.; Lai, L. Chem. Commun. 2018, 54, 3891. |
| [13] | (l) Zhou, K.; Zhang, J.; Qiu, G.; Wu, J. Org. Lett. 2019, 21, 275. |
| [13] | (m) Gong, X.; Li, X.; Xie, W.; Wu, J.; Ye, S. Org. Chem. Front. 2019, 6, 1863. |
| [13] | (n) Wang, X.; Lin, Y.; Liu, J. B. Chin. J. Chem. 2020, 38, 1098. |
| [13] | (o) He, F. S.; Yao, Y.; Xie, W.; Wu, J. Chem. Commun. 2020, 56, 9469. |
| [13] | (p) Zhu, T.; Wu, J. Org. Lett. 2020, 22, 7094. |
| [13] | (q) Huang, J.; Ding, F.; Chen, Z.; Yang, G.; Wu, J. Org. Chem. Front. 2021, 8, 1461. |
| [13] | (r) Yao, Y.; Yin, Z.; He, F. S.; Qin, X.; Xie, W.; Wu, J. Chem. Commun. 2021, 57, 2883. |
| [14] | (a) Zhou, K.; Huang, J.; Wu, J.; Qiu, G. Chin. Chem. Lett. 2021, 32, 37. |
| [14] | (b) Liu, T.; Ding, Y.; Fan, X.; Wu, J. Org. Chem. Front. 2018, 5, 3153. |
| [14] | (c) Ye, S.; Li, X.; Xie, W.; Wu, J. Asian J. Org. Chem. 2019, 8, 893. |
| [14] | (d) Gong, X.; Yang, M.; Liu, J. B.; He, F. S.; Wu, J. Org. Chem. Front. 2020, 7, 938. |
| [14] | (e) Yang, M.; Han, H.; Jiang, H.; Ye, S.; Fan, X.; Wu, J. Chin. Chem. Lett. 2021, DOI: 10.1016j.cclet.2021.05.007. |
| [15] | (a) Wang, X.; Kuang, Y.; Ye, S.; Wu, J. Chem. Commun. 2019, 55, 14962 |
| [15] | (b) Zhu, T.; Shen, J.; Sun, Y.; Wu, J. Chem. Commun. 2021, 57, 915. |
| [16] | (a) Wang, X.; Yang, M.; Xie, W.; Fan, X.; Wu, J. Chem. Commun. 2019, 55, 6010. |
| [16] | (b) Wang, X.; Li, H.; Qiu, G.; Wu, J. Chem. Commun. 2019, 55, 2062. |
| [16] | (c) Gong, X.; Yang, M.; Liu, J. B.; He, F.-S.; Fan, X.; Wu, J. Green Chem. 2020, 22, 1906. |
| [17] | (a) Zhao, Q.; Ren, L.; Hou, J.; Yu, W.; Chang, J. Org. Lett. 2019, 21, 210. |
| [17] | (b) Huang, Z.; Zhang, Q.; Zhao, Q.; Yu, W.; Chang, J. Org. Lett. 2020, 22, 4378. |
| [18] | Zhang, Q.-R.; Xue, D.-Q.; He, P.; Shao, K.-P.; Chen, P.-J.; Gu, Y.-F.; Ren, J.-L.; Shan, L.-H.; Liu, H.-M. Bioorg. Med. Chem. Lett. 2014, 24, 1236. |
| [19] | Ito, S.; Tanaka, Y.; Kakehi, A. Bull. Chem. Soc. Jpn. 1984, 57, 539. |
| [20] | (a) Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O. ACS Catal. 2019, 9, 1103. |
| [20] | (b) Alkan-Zambada, M.; Hu, X. J. Org. Chem. 2019, 84, 4525. |
| [21] | Zhao, Q.; Ren, L.; Hou, J.; Yu, W.; Chang, J. Org. Lett. 2019, 21, 210. |
/
| 〈 |
|
〉 |