

光驱动的金属富勒烯分子磁开关※
收稿日期: 2021-12-15
网络出版日期: 2022-01-20
基金资助
国家自然科学基金(52072374); 国家自然科学基金(51772300); 国家自然科学基金(51832008); 中国科学院青年创新促进会(2018039)
Light-driven Molecular Magnetic Switch for a Metallofullerene※
Received date: 2021-12-15
Online published: 2022-01-20
Supported by
National Natural Science Foundation of China(52072374); National Natural Science Foundation of China(51772300); National Natural Science Foundation of China(51832008); Youth Innovation Promotion Association of CAS(2018039)
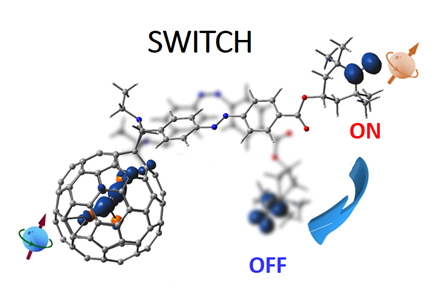
由于碳笼的保护, 从外部操控内嵌富勒烯笼内分子的特性一直是一个挑战. 通过在顺磁性金属富勒烯Sc3C2@C80碳笼外修饰具有光活性的偶氮苯-氮氧自由基, 成功设计出基于金属富勒烯-氮氧自由基的分子开关, 实现了原位可逆地光驱动远程控制金属富勒烯的顺磁特性. 在不同光照条件下, 利用偶氮苯的光异构化特性改变双自旋中心的相对位置, 调整自旋-自旋、自旋-晶格相互作用, 进而影响金属富勒烯的电子顺磁特性. 研究发现, 紫外光照下, 氮氧自由基使金属富勒烯Sc3C2@C80的顺磁信号逐渐减弱, 可见光照下Sc3C2@C80的顺磁信号又增强, 由此实现了氮氧自由基作为顺磁开关的功能.
吴波 , 王冲 , 李宝林 , 王春儒 . 光驱动的金属富勒烯分子磁开关※[J]. 化学学报, 2022 , 80(2) : 101 -104 . DOI: 10.6023/A21120564
Metallofullerene Sc3C2@C80 was synthesized by the arc-discharging method and isolated by multi-stage high performance liquid chromatography. Two Sc3C2@C80 azobenzene nitroxide radical derivatives, compound I and II were synthesized through a Prato reaction, respectively. Usually, azobenzene undergoes trans-cis isomerization when irradiated with light tuned to an appropriate wavelength. The reverse cis-trans isomerization can be driven by light or occurs thermally in the dark. Thus, the compound I was excited by UV light, and it exhibited a strong absorption band decrease at around 340 nm and a slight increase at about 470 nm, which belonging to the π→π* and n→π* transition respectively. Similar to the azobenzene molecule in solution, the typical change of the absorption spectrum of compound I can be ascribed to the trans-to-cis transition with UV light irradiation. Similarly, the reverse isomerization of UV-irradiated compound I with visible light (475 nm) resulted in an obvious π→π* band increase, also indicating the reverse isomerization of compound I from cis-to-trans form. Besides, the structure and spin density distributions of compound I were calculated as well. It has two unpaired spins localizing on the Sc3C2@C80 moiety and nitroxide radical. The magnetic property of metallofullerene can be manipulated by the spin interactions of the two spin centers. The electron paramagnetic resonance (EPR) signals of the trans isomer of the compound I are almost independent of each other. After UV light irradiation, the distance of the two spin centers decreased to r=0.752 nm, and the strong spin-spin interaction weakened the EPR signals of Sc3C2@C80. However, the decreased chain length between Sc3C2@C80 and nitroxide radical would result in a weakened spin-lattice interaction, which increased the EPR signals of the nitroxide radical. Moreover, the UV-radiated compound I with visible light treated later for several minutes, and the EPR signals of Sc3C2@C80 has a certain degree of recovery with visible light irradiation. Therefore, the compound I has sensitive and reversible spin variation with different light irradiation. The remote nitroxide radical group serves as a magnetic switch for the EPR signal of Sc3C2@C80 through the photoisomerization properties of azobenzene bridge. The EPR signals of Sc3C2@C80 moiety were decreased by the strong spin-spin interaction, and the EPR signals of Sc3C2@C80 would be enhanced by larger space with visible light irradiation. Such magnetic switch for metallofullerenes has potential applications in quantum information processing and molecular devices.

| [1] | Popov, A. A.; Yang, S.-F.; Dunsch, L. Chem. Rev. 2013, 113, 5989. |
| [2] | Niu, C.; Wang, G.-W. Chin. J. Org. Chem. 2020, 40, 3633. (in Chinese) |
| [2] | ( 牛闯, 王官武, 有机化学, 2020, 40, 3633.) |
| [3] | Dong, W.; Nie, M.-S.; Lian, Y.-F. Acta Chim. Sinica 2017, 75, 453. (in Chinese) |
| [3] | ( 董薇, 聂梦思, 廉永福, 化学学报, 2017, 75, 453.) |
| [4] | Ma, Y.-H.; Wang, T.-S.; Wu, J.-Y.; Feng, Y.-Q.; Jiang, L.; Shu, C.-Y.; Wang, C.-R. Chem. Commun. 2012, 48, 11570. |
| [5] | Meng, H.-B.; Zhao, C.; Nie, M.-Z.; Wang, C.-R.; Wang, T.-S. Nanoscale 2018, 10, 18119. |
| [6] | Feng, Y.-Q.; Wang, T.-S.; Li, Y.-J.; Li, J.; Wu, J.-Y.; Wu, B.; Jiang, L.; Wang, C.-R. J. Am. Chem. Soc. 2015, 137, 15055. |
| [7] | Yang, S.-F.; Wei, T.; Jin, F. Chem. Soc. Rev. 2017, 46, 5005. |
| [8] | Choi, Y.-J.; Kim, J.-T.; Yoon, W.-J.; Kang, D.-G.; Park, M.; Kim, D.-Y.; Lee, M.-H.; Ahn, S.-k.; Jeong, K.-U. ACS Macro Lett. 2018, 7, 576. |
| [9] | Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Chem. Rev. 2020, 120, 310. |
| [10] | Qian, H.-Y.; He, P.; Zhang, L.; Chen, K.; Xu, B.-B.; Lin, S.-L. Chin. J. Org. Chem. 2021, 41, 2891. (in Chinese) |
| [10] | ( 钱鸿宇, 何品, 张璐, 陈珂, 徐彬彬, 林绍梁, 有机化学, 2021, 41, 2891.) |
| [11] | Zhai, Y.-L.; Xu, W.-J.; Meng, X.-R.; Hou, H.-W. Acta Chim. Sinica 2020, 78, 256. (in Chinese) |
| [11] | ( 翟亚丽, 许文娟, 孟祥茹, 侯红卫, 化学学报, 2020, 78, 256.) |
| [12] | Bandara, H. M. D.; Burdette, S. C. Chem. Soc. Rev. 2012, 41, 1809. |
| [13] | Tavadze, P.; Avendaño Franco, G.; Ren, P.-J.; Wen, X.-D.; Li, Y.-W.; Lewis, J. P. J. Am. Chem. Soc. 2018, 140, 285. |
| [14] | Cheng, S.-C.; Wang, C.-H.; Lin, Y.-C.; Tsuchido, Y.; Suzaki, Y.; Sei, Y.; Kuo, T.-S.; Horie, M. ACS Appl. Mater. Interfaces 2020, 12, 50002. |
| [15] | Ichimura, K.; Oh, S.-K.; Nakagawa, M. Science 2000, 288, 1624. |
| [16] | Shirai, Y.; Sasaki, T.; Guerrero, J. M.; Yu, B.-C.; Hodge, P.; Tour, J. M. ACS Nano 2008, 2, 97. |
| [17] | Lee, T.-H.; Han, G.-Y.; Yi, M.-B.; Kim, H.-J.; Lee, J.-H.; Kim, S. ACS Appl. Mater. Interfaces 2021, 13, 43364. |
| [18] | Yan, Y.; Wang, X.; Chen, J. I. L.; Ginger, D. S. J. Am. Chem. Soc. 2013, 135, 8382. |
| [19] | Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. Nature 1985, 318, 162. |
| [20] | Nakatsuji, S. I.; Fujino, M.; Hasegawa, S.; Akutsu, H.; Yamada, J.-I.; Gurman, V. S.; Vorobiev, A. K. J. Org. Chem. 2007, 72, 2021. |
| [21] | Nachtigall, O.; Lomoth, R.; Dahlstrand, C.; Lundstedt, A.; Gogoll, A.; Webb, M. J.; Grennberg, H. Eur. J. Org. Chem. 2014, 2014, 966. |
| [22] | Kato, T.; Suzuki, S.; Kikuchi, K.; Achiba, Y. J. Phys. Chem. 1993, 97, 13425. |
| [23] | Wang, T.-S.; Wang, C.-R. Acc. Chem. Res. 2014, 47, 450. |
| [24] | Wang, T.-S.; Wu, J.-Y.; Xu, W.; Xiang, J.-F.; Lu, X.; Li, B.; Jiang, L.; Shu, C.-Y.; Wang, C.-R. Angew. Chem., Int. Ed. 2010, 49, 1786. |
| [25] | Li, Y.; Lei, X.; Lawler, R. G.; Murata, Y.; Komatsu, K.; Turro, N. J. J. Phys. Chem. Lett. 2011, 2, 741. |
| [26] | Wu, B.; Wang, T.-S.; Feng, Y.-Q.; Zhang, Z.-X.; Jiang, L.; Wang, C.-R. Nat. Commun. 2015, 6, 6468. |
/
| 〈 |
|
〉 |