

铑催化吲哚C(4)—H选择性活化构建氮杂-螺[4,5]吲哚骨架※
收稿日期: 2021-12-27
网络出版日期: 2022-02-07
基金资助
国家自然科学基金(22001258); 国家自然科学基金(21920102003); 中国科学院青年创新促进会(2018293); 上海市科学技术委员会(17JC1405000); 上海市科学技术委员会(21ZR1475400); 上海市科学技术委员会(18431907100); 上海市学术/技术带头人计划(19XD1424600)
Construction of Aza-spiro[4,5]indole Scaffolds via Rhodium-Catalyzed Regioselective C(4)—H Activation of Indole※
Received date: 2021-12-27
Online published: 2022-02-07
Supported by
National Natural Science Foundation of China(22001258); National Natural Science Foundation of China(21920102003); Youth Innovation Promotion Association CAS(2018293); Science and Technology Commission of Shanghai Municipality(17JC1405000); Science and Technology Commission of Shanghai Municipality(21ZR1475400); Science and Technology Commission of Shanghai Municipality(18431907100); Program of Shanghai Academic Research Leader(19XD1424600)
王孟孟 , 张俊 , 王慧颖 , 马彪 , 戴辉雄 . 铑催化吲哚C(4)—H选择性活化构建氮杂-螺[4,5]吲哚骨架※[J]. 化学学报, 2022 , 80(3) : 277 -281 . DOI: 10.6023/A21120588
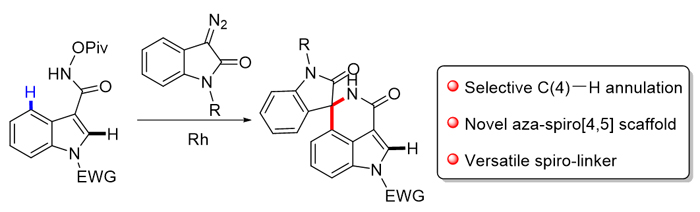
Indole skeletons are widely used in drug research and development as a “privileged structure”, while spirocyclic scaffold as a common structural unit, usually plays an important role in the bioactivity and physicochemical properties of molecule skeletons. Hence, spiroindoles which incorporate both indole and spirocycle units have great significance and widespread applications in pharmaceutical field, and substantial progress has been made in the field for their construction and modification. C—H Activation via directing group's assistance has emerged as a powerful approach in the field of organic synthesis. To date, various 2- and 3-indolyl-tethered aza-spiro-centres via C—H activation have been successfully achieved. However, due to the inherent reactivity of the pyrrole side of the indole, introduction of spiro-containing systems onto the benzenoid core of indole still remains challenging. Here, by installing an appropriate directing group onto the C(3) position of indole, a mild and efficient method of Rh(III)-catalyzed selectively C(4)—H activation/cyclization of indole with diazo compound was developed. As a result, a series of novel aza-spiro[4,5]indole derivatives were obtained in mild to excellent yields. The protocol showed excellent functional group tolerance. Gram-scale synthesis demonstrated the utility of this protocol, and further modification via click chemistry offered the novel scaffold as a versatile spiro linker. A general procedure for the synthesis of spiroindole derivatives is described as the following: to a solution of N-(pivaloyloxy)-indole- 3-carboxamide (0.1 mmol), [Cp*RhCl2]2 (1.6 mg, 2.5 mol%) and NaOAc (1.6 mg, 20 mol%) in CH3CN (1 mL) was added diazooxindole (0.12 mmol) under air. Then the reaction mixture was stirred at room temperature for 12 h. After completion of the reaction, the resulting mixture was diluted with 25 mL of EtOAc, and filtered through a celite pad. Then the filtrate was concentrated under vacuum to give the crude product, which was purified via silica gel to obtain the corresponding spiroindole product.
Key words: indole spirocycle; C—H activation; Rh-catalyst
| [1] | For selected reviews: (a) Chauhan, M.; Saxena, A.; Saha, B.. Eur. J. Med. Chem. 2021, 218, 113400. |
| [1] | (b) Thanikachalam, P. V.; Maurya, R. K.; Garg, V.; Monga, V. Eur. J. Med. Chem. 2019, 180, 562. |
| [1] | (c) Sravanthi, T. V.; Manju, S. L. Eur. J. Pharm. Sci. 2016, 91, 1. |
| [1] | (d) Somei, M.; Yamada, F. Nat. Prod. Rep. 2005, 22, 73. |
| [1] | (e) Kochanowska-Karamyan, A. J.; Hamann, M. T. Chem. Rev. 2010, 110, 4489. |
| [2] | For selected examples: (a) Hiesinger, K.; Dar'in, D.; Proschak, E.; Krasavin, M. J. Med. Chem. 2021, 64, 150. |
| [2] | (b) Ghatpande, N. G.; Jadhav, J. S.; Kaproormath, R. V.; Soliman, M. E.; Shaikh, M. M. Bioorg. Med. Chem. 2020, 28, 115813. |
| [2] | (c) Ren, B.-Y.; Yi, J.-C.; Zhong, D.-K.; Zhao, Y.-Z.; Guo, R.-D.; Sheng, Y.-G.; Sun, Y.-G.; Xie, L.-H.; Huang, W. Acta Chim. Sinica 2020, 78, 56. (in Chinese) |
| [2] | (任保轶, 依建成, 钟道昆, 赵玉志, 郭闰达, 盛永刚, 孙亚光, 解令海, 黄维, 化学学报, 2020, 78, 56.) |
| [2] | (d) Benabdallaha, M.; Talhib, O.; Noualia, F.; Choukchou-Brahama, N.; Bacharib, K.; Silva, A. M. S. Curr. Med. Chem. 2018, 25, 3748. |
| [3] | For selected examples: (a) Nasri, S.; Bayat, M.; Mirzaei, F. Top. Curr. Chem. 2021, 379, 25. |
| [3] | (b) Bora, D.; Kaushal, A.; Shankaraiah, N. Eur. J Med. Chem. 2021, 215, 113263. |
| [3] | (c) Li, B.; Zhou, R.; He, G.; Guo, L.; Huang, W. Acta Chim. Sinica 2013, 71, 1396. (in Chinese) |
| [3] | (李博, 周锐, 何谷, 郭丽, 黄维, 化学学报, 2013, 71, 1396.) |
| [4] | For selected examples: (a) Zhou, B.; Liang, R.; Cao, Z.; Zhou, P.; Jia, Y. Acta Chim. Sinica 2021, 79, 176 (in chinses). |
| [4] | (周波, 梁仁校, 曹中艳, 周平海, 贾义霞, 化学学报, 2021, 79, 176.) |
| [4] | (b) Li, N.-K.; Zhang, J.-Q.; Sun, B.-B.; Li, H.-Y.; Wang, X.-W. Org. Lett. 2017, 19, 1954. |
| [4] | (c) Chen, X.-Y.; Baratay, C. A.; Mark, M. E.; Xu, X.-F.; Chan, P. W. H. Org. Lett. 2020, 22, 2849. |
| [4] | (d) Zhu, M.; Zheng, C.; Zhang, X.; You, S.-L. J. Am. Chem. Soc. 2019, 141, 2636. |
| [4] | (e) Tang, S.; Wang, J.; Xiong, Z.; Xie, Z.; Li, D.; Huang, J.; Zhu, Q. Org. Lett. 2017, 19, 5577. |
| [4] | (f) Qiu, H.; Zhang, D.; Liu, S.; Qiu, L.; Zhou, J.; Qian, Y.; Zhai, C.; Hu, W. Acta Chim. Sinica. 2012, 70, 2484. (in Chinese) |
| [4] | (邱晃, 张丹, 刘顺英, 邱林, 周俊, 钱宇, 翟昌伟, 胡文浩, 化学学报, 2012, 70, 2484.) |
| [5] | For selected reviews: (a) Sinha, S. K.; Guin, S.; Maiti, S.; Biswas, J. P.; Porey, S.; Maiti, D. Chem. Rev. 2021, DOI: 10.1021/acs.chemrev.1c00220. |
| [5] | (b) Zhu, W.-H.; Gunnoe, T. B. Acc. Chem. Res. 2020, 53, 920. |
| [5] | (c) Rej, S.; Chatani, N. Angew. Chem., Int. Ed. 2019, 58, 8304. |
| [5] | (d) Hong, J.; Li, M.; Zhang, J.; Sun, B.; Mo, F. ChemSusChem 2019, 12, 6. |
| [5] | (e) Chu, J. C. K.; Rovis, T. Angew. Chem., Int. Ed. 2018, 57, 62. |
| [5] | (f) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754. |
| [5] | (g) Zhu, R.-Y.; Farmer, M. E.; Chen, Y.-Q.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 10578. |
| [5] | (h) Zhao, J.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235. (in Chinese) |
| [5] | (赵金钵, 张前, 化学学报, 2015, 73, 1235.) |
| [6] | (a) Zeng, H.-Y.; Wang, Z.-M.; Li, C.-J. Angew. Chem., Int. Ed. 2019, 58, 2859. |
| [6] | (b) Wang, Z.-M.; Niu, J.-B.; Zeng, H.-Y.; Li, C.-J. Org. Lett. 2019, 21, 7033. |
| [6] | (c) Zheng, J.; Zhang, Y.; Cui, S.-L. Org. Lett. 2014, 16, 3560. |
| [6] | (d) Chabaud, L.; Raynal, Q.; Barre, E.; Guillou, C. Adv. Synth. Catal. 2015, 357, 3880. |
| [7] | Zhang, Y.; Zheng, J.; Cui, S.-L. J. Org. Chem. 2014, 79, 6490. |
| [8] | Zhang, J.; Wang, M.; Wang, H.; Xu, H.; Chen, J.; Guo, Z.; Ma, B.; Ban, S.-R.; Dai, H.-X. Chem. Commun. 2021, 57, 8656. |
| [9] | (a) Kumar, P.; Nagtilak, P. J.; Kapur, M. New J. Chem. 2021, 45, 13692. |
| [9] | (b) Kalepu, J.; Gandeepan, P.; Ackermann, L.; Pilarski, L. T. Chem. Sci. 2018, 9, 4203. |
| [10] | For selected reviews: (a) Wen, J.; Shi, Z. Acc. Chem. Res. 2021, 54, 1723. |
| [10] | (b) Prabagar, B.; Yang, Y.; Shi, Z. Chem. Soc. Rev. 2021, 50, 11249. |
| [11] | For selected examples: (a) Kuang, G.; Liu, D.; Chen, X.; Liu, G.; Fu, Y.; Peng, Y.; Li, H.; Zhou, Y. Org. Lett. 2021, 23, 8402. |
| [11] | (b) Lanke, V.; Prabhu, K. R. Chem. Commun. 2017, 53, 5117. |
| [11] | (c) Liu, X.; Li, G.; Song, F.; You, J. Nat. Commun. 2014, 5, 5030. |
| [11] | (d) Chen, S.; Feng, B.; Zheng, X.; Yin, J.; Yang, S.; You, J. Org. Lett. 2017, 19, 2502. |
| [12] | For selected examples: (a) Cheng, Y.; Yu, S.; He, Y.; An, G.; Li, G.; Yang, Z. Chem. Sci. 2021, 12, 3216. |
| [12] | (b) Banjare, S. K.; Nanda, T.; Pati, B. V.; Das Adhikari, G. K.; Dutta, J.; Ravikumar, P. C. ACS Catal. 2021, 11, 11579. |
| [12] | (c) Harada, S.; Yanagawa, M.; Nemoto, T. ACS Catal. 2020, 10, 11971. |
| [12] | (d) Pradhan, S.; Mishra, M.; Bhusan De, P.; Banerjee, S.; Punniyamurthy, T. Org. Lett. 2020, 22, 1720. |
| [12] | (e) Lv, J.; Chen, X.; Xu, X.-S.; Zhao, B.; Liang, Y.; Wang, M.; Jin, L.; Yuan, Y.; Han, Y.; Zhao, Y.; Lu, Y.; Zhao, J.; Sun, W.-Y.; Houk, K. N.; Shi, Z. Nature 2019, 575, 336. |
| [12] | (f) Yang, Y.; Gao, P.; Zhao, Y.; Shi, Z. Angew. Chem., Int. Ed. 2017, 56, 3966. |
| [12] | (g) Sherikar, M. S.; Kapanaiah, R.; Lanke, V.; Prabhu, K. R. Chem. Commun. 2018, 54, 11200. |
| [12] | (h) Zhang, J.; Wu, M.; Fan, J.; Xu, Q.; Xie, M. Chem. Commun. 2019, 55, 8102. |
| [12] | (i) Banjare, S. K.; Nanda, T.; Ravikumar, P. C. Org. Lett. 2019, 21, 8138. |
| [13] | Maity, S.; Karmakar, U.; Samanta, R. Chem. Commun. 2017, 53, 12197. |
| [14] | For selected examples: (a) Zhang, Q.; Xie, X.; Peng, J.; Chen, F.; Ma, J.; Li, C.; Liu, H.; Wang, D.; Wang, J. Org. Lett. 2021, 23, 4699. |
| [14] | (b) Bai, Z.; Cai, C.; Sheng, W.; Ren, Y.; Wang, H. Angew. Chem., Int. Ed. 2020, 59, 14686. |
| [14] | (c) Liu, Q.; Li, Q.; Ma, Y.; Jia, Y. Org. Lett. 2013, 15, 4528. |
| [14] | (d) Ge, Y.; Wang, H.; Wang, H.-N.; Yu, S.-S.; Yang, R.; Chen, X.; Zhao, Q.; Chen, G. Org. Lett. 2021, 23, 370. |
| [15] | For selected examples: (a) Kona, C. N.; Nishii, Y.; Miura, M. Angew. Chem., Int. Ed. 2019, 58, 9856. |
| [15] | (b) Kona, C. N.; Nishii, Y.; Miura, M. Org. Lett. 2018, 20, 4898. |
/
| 〈 |
|
〉 |