

五氧化二钒促进MgH2/Mg室温吸氢※
收稿日期: 2021-12-14
网络出版日期: 2022-02-08
基金资助
山东省重点研发计划(2020CXGC010402); 国家自然科学基金(51801197); 中国科学院青年创新促进会(2019189); 辽宁省自然科学基金(2021-BS-010)
Room Temperature Hydrogen Absorption of V2O5 Catalyzed MgH2/Mg※
Received date: 2021-12-14
Online published: 2022-02-08
Supported by
Key R&D Program of Shandong Province(2020CXGC010402); National Natural Science Foundation of China(51801197); Youth Innovation Promotion Association of Chinese Academy of Science(2019189); Natural Science Foundation of Liaoning Province(2021-BS-010)
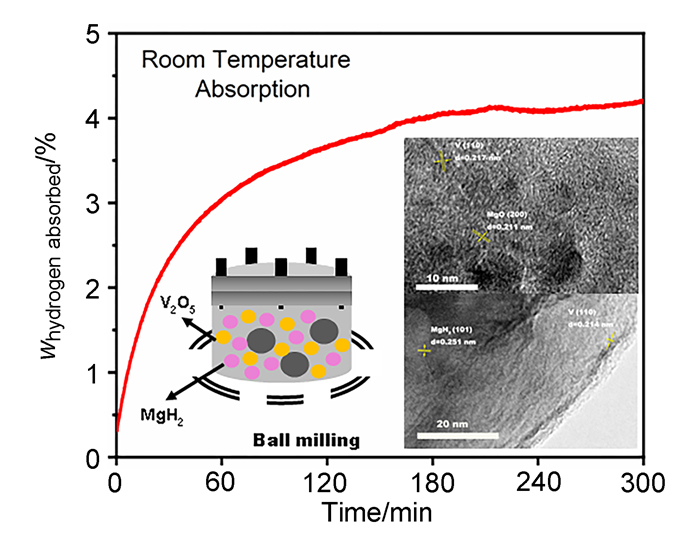
MgH2因其储氢量高、来源广及价格低廉等优点而备受关注, 但其热力学稳定(ΔH≥76 kJ/mol-H2)以及低温吸/放氢动力学缓慢等问题限制了它在氢能领域的广泛应用. 研究发现, 过渡金属氧化物能够显著改善MgH2的储氢动力学性能. 系统研究了过渡金属氧化物V2O5对MgH2储氢性能的改善作用. 与纯MgH2相比, 在MgH2中添加质量分数为5%的V2O5可以显著改善MgH2的吸/脱氢动力学性能. V2O5掺杂MgH2的起始脱氢温度降至175 ℃, 比同等条件处理的纯MgH2降低了89 ℃. 值得注意的是, V2O5掺杂的MgH2脱氢后, 在室温和3 MPa的氢压下, 30和180 min内吸收H2的质量分数分别为2.1%和3.8%. 同等氢压下, 当温度提高到300 ℃时, 该样品可在1 min内吸收H2的质量分数高达6.7%. 同时催化掺杂样品还表现出良好的循环稳定性, 20次循环后仍能维持质量分数为6.0%以上的可逆储/放氢量. 此外, V2O5改善MgH2储氢性能的反应机理也通过多种手段表征得以阐明.
戴敏 , 雷钢铁 , 张钊 , 李智 , 曹湖军 , 陈萍 . 五氧化二钒促进MgH2/Mg室温吸氢※[J]. 化学学报, 2022 , 80(3) : 303 -309 . DOI: 10.6023/A21120561
Magnesium hydride is a promising hydrogen storage material due to its high hydrogen storage capacity, low cost and abundance. The gravimetric and volumetric hydrogen capacities of MgH2 are about 7.6% and 110 g/L, respectively. However, its sluggish de/re-hydrogenation rates and high operating temperatures ranging between 300~400 ℃ restrict it in practical applications. Catalyzing has been proved to be an effective method to improve its hydrogen storage performance. In this work, V2O5 has been chosen as a catalyst for improving the de/re-hydrogenation kinetics of MgH2. Experimental results show that MgH2 doping with V2O5 (w=5%) has the best hydrogen storage properties among the doping amounts (w) of 2.5% to 10%. Comparing with the pristine MgH2, the addition of V2O5 (w=5%) significantly improves the ab/desorption behaviors of MgH2. V2O5 (w=5%) doped MgH2 starts releasing hydrogen from 175 ℃ which is 89 ℃ lower than the additive-free as-milled MgH2. It should be noted that the dehydrogenated V2O5 (w=5%) doped MgH2, is able to absorb 2.1% and 3.8% in the mass fraction of H2 respectivity, within 30 and 180 min at room temperature and 3 MPa hydrogen pressure. Under the same hydrogen pressure, when the temperature is increased to 300 ℃, the mass fraction of H2 absorbed by the sample is as high as 6.7% within 1 min. In addition, the catalyzed system shows a good reversibility, after 20 cycles, the hydrogen capacity maintains above 6.0%. Compared with the pure MgH2, the dehydrogenation apparent activation energy of V2O5 catalyzed sample decreased from 108 to 56 kJ•mol–1. X-Ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) have been employed to investigate its reaction mechanism. It shows that the formation of metallic vanadium and low-oxidation vanadium during ball milling and dehydrogenation process play important roles in improving the de/re-hydrogenation kinetics of MgH2/Mg system.

| [1] | Lee, D.-H.; Hung, C.-P. Int. J. Hydrogen Energy 2012, 37, 15753. |
| [2] | Endo, N.; Goshome, K.; Tetsuhiko, M.; Segawa, Y.; Shimoda, E.; Nozu, T. Int. J. Hydrogen Energy 2021, 46, 262. |
| [3] | Guo, L. Chem. Eng. Des. Commun. 2021, 47, 147. (in Chinese) |
| [3] | (郭利, 化工设计通讯, 2021, 47, 147.) |
| [4] | Wang, F.; Harindintwali, J. D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; Chang, S. X.; Zhang, L.; Rinklebe, J.; Yuan, Z.; Zhu, Q.; Xiang, L.; Tsang, D. C. W.; Xu, L.; Jiang, X.; Liu, J.; Wei, N.; Kästner, M.; Zou, Y.; Ok, Y. S.; Shen, J.; Peng, D.; Zhang, W.; Barceló, D.; Zhou, Y.; Bai, Z.; Li, B.; Zhang, B.; Wei, K.; Cao, H.; Tan, Z.; Zhao, L.-B.; He, X.; Zheng, J.; Bolan, N.; Liu, X.; Huang, C.; Dietmann, S.; Luo, M.; Sun, N.; Gong, J.; Gong, Y.; Brahushi, F.; Zhang, T.; Xiao, C.; Li, X.; Chen, W.; Jiao, N.; Lehmann, J.; Zhu, Y.-G.; Jin, H.; Schäffer, A.; Tiedje, J. M.; Chen, J. M. The Innovation 2021, 2, 100180. |
| [5] | Pistidda, C. Hydrogen 2021, 2, 428. |
| [6] | Xiao, G. P.; Qiao, W. J.; Zhang, L.; Qing, S. J.; Zhang, C. S.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 100. (in Chinese) |
| [6] | (肖国鹏, 乔韦军, 张磊, 庆绍军, 张财顺, 高志贤, 化学学报, 2021, 79, 100.) |
| [7] | Jiao, T.; Xu, X. L.; Zhang, L.; Weng, Y. Y.; Weng, Y. B.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 513. (in Chinese) |
| [7] | (焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.) |
| [8] | Chen, Y. J. Coal Chem. Ind. 2020, 43, 130. (in Chinese) |
| [8] | (陈英杰, 煤炭与化工, 2020, 43, 130.) |
| [9] | Hu, S. X.; Yu, Z. L.; Li, C. Y.; Wang, Z. Q.; Guo, S.; Huang, R. J.; Fang, Y. T. J. Fuel Chem. Technol. 2015, 43, 385. (in Chinese) |
| [9] | (胡顺轩, 余钟亮, 李春玉, 王志青, 郭帅, 黄戒介, 房倚天, 燃料化学学报, 2015, 43, 385.) |
| [10] | Zhang, Y.; Wang, S. X.; Yang, R.; Dai, T. Y.; Zhang, N.; Xi, P. X.; Yan, C. H. Acta Chim. Sinica 2020, 78, 1455. (in Chinese) |
| [10] | (张宇, 王世兴, 杨蕊, 戴腾远, 张楠, 席聘贤, 严纯华, 化学学报, 2020, 78, 1455.) |
| [11] | Ma, W. P.; He, Y. Y.; Liu, H. L. Acta Chim. Sinica 2021, 79, 914. (in Chinese) |
| [11] | (麻旺坪, 贺彦彦, 刘洪来, 化学学报, 2021, 79, 914.) |
| [12] | Feng, X.; Yang, Z. H.; CHENDe. Chem. Ind. Eng. Prog. Doi: 10.16085/j.issn.1000-6613.2021-2233. (in Chinese) |
| [12] | (冯翔, 杨朝合, CHENDe, 化工进展, Doi: 10.16085/j.issn.1000-6613.2021-2233.) |
| [13] | Eberle, U.; Felderhoff, M.; Schüth, F. Angew. Chem. 2009, 48, 6608. |
| [14] | Schlapbach, L.; Züttel, A. Nature 2001, 414, 353. |
| [15] | Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X. J. Research 2021, 2021, 3750689. |
| [16] | Lim, K. L.; Kazemian, H.; Yaakob, Z.; Daud, W. R. W. Chem. Eng. Technol. 2010, 33, 213. |
| [17] | Ma, T. X.; Gao, L. Z.; Hu, M. J.; Hu, L. W.; Wen, L. Y.; Hu, M. L. J. Funct. Mater. 2018, 49, 4001. (in Chinese) |
| [17] | (马通祥, 高雷章, 胡蒙均, 胡丽文, 温良英, 扈玫珑, 功能材料, 2018, 49, 4001.) |
| [18] | Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Energy Storage Mater. 2018, 10, 168. |
| [19] | Zhang, Q. Y.; Du, S. C.; Ma, Z. W.; Lin, X.; Zou, J. X.; Zhu, W.; Ren, L.; Li, Y. H. Chin. Sci. Bull. 2021, 66, 1 (in Chinese) |
| [19] | (张秋雨, 杜四川, 马哲文, 林羲, 邹建新, 朱文, 任莉, 李映辉, 科学通报, 2021, 66, 1.) |
| [20] | Fernández, J. F.; Sánchez, C. R. J. Alloys Compd. 2002, 340, 189. |
| [21] | Huot, J.; Tremblay, M. L.; Schulz, R. J. Alloys Compd. 2003, 356, 603. |
| [22] | Bobet, J. L.; Desmoulins-Krawiec, S.; Grigorova, E.; Cansell, F.; Chevalier, B. J. Alloys Compd. 2003, 351, 217. |
| [23] | Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Energy Environ. Sci. 2021, 14, 2302. |
| [24] | Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 292, 247. |
| [25] | Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 315, 237. |
| [26] | Pelletier, J. F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A. R.; Lurio, L. B.; Mochrie, S. G. J. Phys. Rev. B 2001, 63, 052103. |
| [27] | Luo, B.; Yao, Z.; Xiao, X.; Hang, Z.; Jiang, F.; Liu, M.; Chen, L. Mater. Chem. Phys. 2021, 263, 124342. |
| [28] | Hu, S.; Zhang, H.; Yuan, Z.; Wang, Y.; Fan, G.; Fan, Y.; Liu, B. J. Alloys Compd. 2021, 881, 160571. |
| [29] | Adedeji Bolarin, J.; Zhang, Z.; Cao, H.; Li, Z.; He, T.; Chen, P. J. Phys. Chem. C 2021, 125, 19631. |
| [30] | Zhang, J.; Huang, Y. N.; Mao, C.; Long, C. G.; Shao, Y. M.; Fu, J. Q.; Peng, P. Acta Chim. Sinica 2010, 68, 2077. (in Chinese) |
| [30] | (张健, 黄雅妮, 毛聪, 龙春光, 邵毅敏, 付俊庆, 彭平, 化学学报, 2010, 68, 2077.) |
| [31] | Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.-W.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; Horita, Z. Acta Mater. 2018, 149, 88. |
| [32] | Nyamsi, S. N.; Wu, Z.; Guo, L.; Qian, C.; Sun, L.; Xu, F.; Uesugi, H.; Yan, G.; Yang, F.; Zhang, Z. ACS Appl. Energy Mater. 2021, 4, 5973. |
| [33] | Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. J. Power Sources 2018, 396, 796. |
| [34] | Liao, W.; Jiang, W.; Yang, X.-S.; Wang, H.; Ouyang, L.; Zhu, M. J. Rare Earths 2021, 39, 1010. |
| [35] | Kato, S.; Borgschulte, A.; Bielmann, M.; Züttel, A. Phys. Chem. Chem. Phys. 2012, 14, 8360. |
| [36] | Chu, H.; Qiu, S.; Sun, L.; Huot, J. Dalton Trans. 2015, 44, 16694. |
| [37] | Yu, H.; Bennici, S.; Auroux, A. Int. J. Hydrogen Energy 2014, 39, 11633. |
| [38] | Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. ACS Appl. Mater. Interfaces 2021, 13, 13235. |
| [39] | Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. J. Alloys Compd. 2016, 669, 8. |
| [40] | Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 291, 295. |
| [41] | Hanada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fujii, H.; Kojima, Y. J. Phys. Chem. C 2009, 113, 13450. |
| [42] | Barkhordarian, G.; Klassen, T.; Bormann, R. J. Phys. Chem. B 2006, 110, 11020. |
| [43] | Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 322, L5. |
| [44] | Korablov, D.; Nielsen, T. K.; Besenbacher, F.; Jensen, T. R. Powder Diffr. 2015, 30, S9. |
/
| 〈 |
|
〉 |