

基于层状氢氧化镧的稀土离子激活杂化组装荧光体
收稿日期: 2021-10-27
网络出版日期: 2021-11-16
基金资助
国家自然科学基金(51972097)
Rare Earth Ions-Activated Hybrid Assemblies Fluorescent Systems Based on the Layered Lanthanum Hydroxides
Received date: 2021-10-27
Online published: 2021-11-16
Supported by
National Natural Science Foundation of China(51972097)
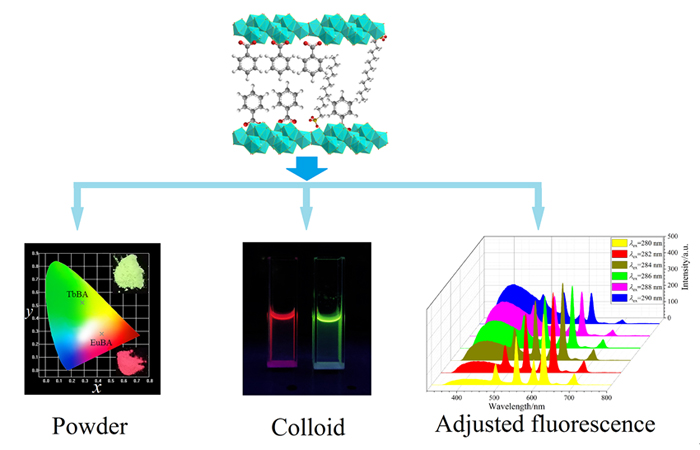
采用水热法制备了十二烷基磺酸根(DS-)插层的稀土离子(Eu3+, Tb3+和Ce3+)激活的层状氢氧化镧(LLaH), 通过微波法将苯甲酸根(BA-)与层中DS-进行离子交换反应, 形成杂化组装. X-射线衍射(XRD)结果表明这些杂化组装具有典型的层状结构, 离子交换反应后层间距由3.2 nm减小为1.9 nm. 光致发光光谱显示稀土Eu3+和Tb3+掺杂的LLaH均表现出相应的特征红色和绿色发射, 但(Eu/Tb)0.1La1.9(OH)5BA•H2O的发光强度是(Eu/Tb)0.1La1.9(OH)5DS•H2O的十几倍, 这得益于BA-对稀土离子发光产生了很好的敏化作用. 通过超声和离心过程将Tb0.1La1.9(OH)5BA•H2O, Eu0.1La1.9(OH)5BA•H2O和Ce0.08La1.92(OH)5DS•H2O杂化组装样品进行层剥离制成胶体溶液, 发现不同比例的Eu0.1La1.9(OH)5BA•H2O和Tb0.1La1.9(OH)5BA•H2O两种胶体混合能够对发光颜色进行调整; 三种胶体混合后通过改变激发波长也可以有效调整发光颜色, 特别是在280~290 nm紫外光激发下, 能够获得白色荧光, 显示出优异的光功能特性.
左翔宇 , 许怡飞 , 石士考 . 基于层状氢氧化镧的稀土离子激活杂化组装荧光体[J]. 化学学报, 2022 , 80(2) : 133 -140 . DOI: 10.6023/A21100484
The layered rare earth hydroxides (LRHs) have recently been paid attention owing to the adjustable rare earth ions in the host and alterable anions in the gallery, which may be applied in adsorption, catalyst, fluorescence and detection fields. However, as far as we know, the studies of rare earth ions-activated layered lanthanum hydroxide (LLaH) fluorescent systems were seldom reported. In this paper, the hybrid assemblies (Eu/Tb)0.1La1.9(OH)5DS•H2O and Ce0.08La1.92(OH)5DS• H2O solid samples were first prepared with Eu(NO3)3, TbCl3, Ce(NO3)3•6H2O and La(NO3)3 solutions by hydrothermal process at 140 ℃ for 12 h, in which the interlayer was pillared with dodecyl sulfonate (DS-) anions. Then, the DS- anions of the as-prepared (Eu/Tb)0.1La1.9(OH)5DS•H2O were exchanged with benzoate (BA-) solution through microwave process, and the hybrid assemblies samples (Eu/Tb)0.1La1.9(OH)5BA•H2O were obtained. The X-ray diffraction (XRD) results indicate that the solid samples have superior crystallization and layered structure, and the interlayer distance is decreased from 3.2 nm to 1.9 nm after the ion-exchange reaction. The photoluminescence excitation spectra show that the excitation intensities in the ultraviolet (UV) region (≈280 nm) for (Eu/Tb)0.1La1.9(OH)5BA•H2O are much stronger than those for (Eu/Tb)0.1La1.9- (OH)5DS•H2O due to the strong absorption peak from BA- anion. Excited with 280 nm, the emission spectra exhibit the characteristic emission transitions of Eu3+ or Tb3+ ions, and the intensities of (Eu/Tb)0.1La1.9(OH)5BA•H2O are more than tenfold of (Eu/Tb)0.1La1.9(OH)5DS•H2O because of efficient sensitization effect of BA-. On the other hand, the (Eu/Tb)0.1La1.9(OH)5BA•H2O and Ce0.08La1.92(OH)5DS•H2O hybrid assemblies were exfoliated to colloidal solutions through ultrasound and centrifugation process in formamide solvent. It has been observed that the fluorescent color can be adjusted by mixing the colloid solutions of Eu0.1La1.9(OH)5BA•H2O and Tb0.1La1.9(OH)5BA•H2O in different volume ratios. Moreover, the fluorescent color can be changed with the three colloid components by varying the excitation wavelength. In particular, the white light can be achieved as excited with 280—290 nm UV light, exhibiting supreme photofunctional performances. The investigation will promote the effective combination of rare earth luminescence with layered materials.

Key words: layered hydroxide; rare earth; fluorescence; luminescent colloid; ion-exchange
| [1] | Liu, J.; Kaczmarek, A. M.; Deun, R. V. Chem. Soc. Rev. 2018, 47, 7225. |
| [2] | Qin, X.; Liu, X. W.; Huang, W.; Bettinelli, M.; Liu, X. G. Chem. Rev. 2017, 117, 4488. |
| [3] | Yang, Y. S.; Wang, K. Z.; Yan, D. P. ACS Appl. Mater. Interfaces 2017, 9, 17399. |
| [4] | Yang, X. G.; Lin, X. Q.; Zhao, Y. B.; Zhao, Y. S.; Yan, D. P. Angew. Chem., Int. Ed. 2017, 56, 7853. |
| [5] | Yang, Y.-S.; Wang, K.-Z.; Zhao, Z.; Yan, D.-P. J. Chin. Soc. Rare Earths 2021, 39, 1. (in Chinese) |
| [5] | ( 杨永晟, 王克志, 赵震, 闫东鹏, 中国稀土学报, 2021, 39, 1.) |
| [6] | Gao, R.; Zhao, M. J.; Guan, Y.; Fang, X. Y.; Li, X. H.; Yan, D. P. J. Mater. Chem. C 2014, 2, 9579. |
| [7] | Gao, R.; Kodaimati, M. S.; Yan, D. P. Chem. Soc. Rev. 2021, 50, 5564. |
| [8] | Yapryntsev, A.; Abdusatorov, B.; Yakushev, I.; Svetogorov, R.; Gavrikov, A.; Rodina, A.; Fatyushina, Y.; Baranchikov, A.; Zubavichus, Y.; Ivanov, V. Dalton Trans. 2019, 48, 6111. |
| [9] | Jung, H.; Kim, H.; Byeon, S. H. ACS Appl. Mater. Interfaces 2018, 10, 43112. |
| [10] | Shao, B. Y.; Zhang, X. B.; Sang, S.; Guo, A. P.; Cui, F. M.; Yang, X. J. Eur. Polym. J. 2021, 147, 110324. |
| [11] | Kim, H.; Lee, B. I.; Jeong, H.; Byeon, S. H. J. Mater. Chem. C 2015, 3, 7437. |
| [12] | Jeong, H.; Lee, B. I.; Byeon, S. H. ACS Appl. Mater. Interfaces 2016, 8, 10946 |
| [13] | Wang, B.; Xia, J. F.; Zhou, G. H.; Li, X.; Dai, M. T.; Jiang, D. Y.; Li, Q. RSC Adv. 2020, 10, 37500. |
| [14] | Shao, B. Y.; Zhang, X. B.; Wang, X. Y.; Cui, F. M.; Yang, X. J. Opt. Mater. 2020, 100, 109597. |
| [15] | Pereira, C. C. L.; Lima, J. C.; Moro, A. J.; Monteiro, B. Appl. Clay Sci. 2017, 146, 216. |
| [16] | Sokolov, M. R.; Enakieva, Y. Y.; Yapryntsev, A. D.; Shiryaev, A. A.; Zvyagina, A. I.; Kalinina, M. A. Adv. Funct. Mater. 2020, 30, 2000681. |
| [17] | Gu, Q. Y.; Li, J. Y.; Ji, L. S.; Ju, R. J.; Jin, H. B.; Zhang, R. Y. Front. Mater. Sci. 2020, 14, 488. |
| [18] | Chen, Y. F.; Zhang, Y. J.; Zhang, J. W.; Wang, L. Luminescence 2020, 35, 1125. |
| [19] | Jeon, H. G.; Kim, H.; Byeon, S. H. Chem. Eng. J. 2021, 405, 126675. |
| [20] | Wu, L. Y.; Chen, G. M.; Li, Z. B. Small 2017, 13, 1604070. |
| [21] | Wu, L. Y.; Gao, C. Y.; Li, Z. B.; Chen, G. M. J. Mater. Chem. C 2017, 5, 5207. |
| [22] | Su, F. F.; Guo, R.; Yu, Z. H.; Li, J.; Liang, Z. P.; Shi, K. R.; Ma, S. L.; Sun, G. B.; Li, H. F. Dalton Trans. 2018, 47, 5380. |
| [23] | Zhu, Q.; Li, J. G.; Zhi, C.; Ma, R.; Sasaki, T.; Xu, J. X.; Liu, C. H.; Li, X. D.; Sun, X. D.; Sakka, Y. J. Mater. Chem. 2011, 21, 6903. |
| [24] | McIntyre, L. J.; Jackson, L. K.; Fogg, A. M. J. Phys. Chem. Solids 2008, 69, 1070. |
| [25] | Jeon, H. G.; Kim, H.; Byeon, S. H. Adv. Mater. Interfaces 2019, 1901385. |
| [26] | Liu, W. D.; Zhang, J.; Yin, X. B.; He, X. Y.; Wang, X. P.; Wei, Y. Z. Mater. Chem. Phys. 2021, 266, 124540. |
| [27] | Ma, H.; Li, X.-X.; Sun, Y.-H.; Wang, J.; Chu, N.-K.; Ma, S.-L. J. Beijing Norm. Univ. (Nat. Sci.) 2011, 47, 607. (in Chinese) |
| [27] | ( 马辉, 李新新, 孙亚红, 王娟, 楚楠凯, 马淑兰, 北京师范大学学报(自然科学版), 2011, 47, 607.) |
| [28] | Xu, J.-J.; Zhang, Q.-M.; Jin, Z.; Chu, H.-B.; Li, Y. Chin. J. Inorg. Chem. 2006, 22, 1411. (in Chinese) |
| [28] | ( 许军舰, 张庆敏, 金钟, 褚海斌, 李彦, 无机化学学报, 2006, 22, 1411.) |
| [29] | Hou, X.-H.; Xu, G.; Wei, X.; Han, G.-R. Rare Met. Mater. Eng. 2008, 37, 437. (in Chinese) |
| [29] | ( 侯晓红, 徐刚, 魏晓, 韩高荣, 稀有金属材料与工程, 2008, 37, 437.) |
| [30] | Anchalee, W.; Franziska, S.; Warner, J. H.; Dermot, O. J. Mater. Chem. 2012, 22, 7751. |
| [31] | Zhang, K.-L.; Yuan, J.-B.; Yuan, L.-J.; Sun, J.-T. Acta Chim. Sinica 2000, 58, 144. (in Chinese) |
| [31] | ( 张克立, 袁继兵, 袁良杰, 孙聚堂, 化学学报, 2000, 58, 144.) |
| [32] | Yuan, L.; Sun, J.; Zhang, K. Spectrochim. Acta, Part A 2003, 59, 729. |
| [33] | Utochnikova, V. V.; Kuzmina, N. P. Russ. J. Coord. Chem. 2016, 42, 679. |
| [34] | Zhong, S. L.; Zhang, L. F.; Jiang, J. W.; Lv, Y. H.; Xu, R.; Xu, A. W.; Wang, S. P. CrystEngComm 2011, 13, 4151. |
| [35] | Liu, L.; Yu, J. J.; Shi, S. K.; Wang, J. Y.; Song, H. H.; Zhang, R. K.; Fu, L. S. J. Rare Earths, https://doi.org/10.1016/j.jre.2021.07.011. |
/
| 〈 |
|
〉 |