

钾位铈掺杂黄钾铁矾的磷吸附特性及机理研究※
收稿日期: 2021-12-30
网络出版日期: 2022-03-04
基金资助
中国科学院青年创新促进会专项基金(2021302); 中国科学院青年创新促进会专项基金(2021305); 中国科学院福建物质结构研究所与中国科学院城市环境研究所融合发展基金培育项目(RHZX-2019-003)
K+-Site Ce-Doped Jarosite for Phosphate Adsorption: a Mechanism Study※
Received date: 2021-12-30
Online published: 2022-03-04
Supported by
Youth Innovation Promotion Association CAS(2021302); Youth Innovation Promotion Association CAS(2021305); FJIRSM&IUE Joint Research Fund(RHZX-2019-003)
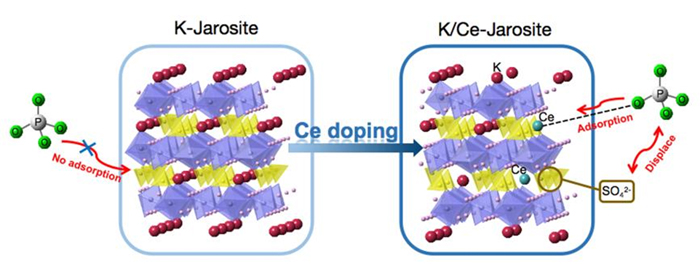
黄钾铁矾是一种自然界常见的含铁矿物, 它对砷酸根有一定吸附作用, 但几乎不吸附同样构型的磷酸根. 为了改善黄钾铁矾的磷吸附性质, 本研究制备了铈掺杂的黄钾铁矾, 并采用X射线衍射(XRD)、电感耦合等离子发射光谱(ICP-OES)等表征手段, 构建了铈离子占据钾离子位的结构模型. 磷吸附实验结果表明, 少量铈的掺杂可将黄钾铁矾的磷吸附容量(pH=7, 24 h)从1.69 mg/g显著提升至29.33 mg/g. 同时, 初始pH和共存阴离子对其除磷效果影响较小, 说明含铈黄钾铁矾对磷酸盐的吸附具备高选择性. 进一步分析表明, 该吸附过程符合准二级动力学模型, 吸附等温线符合Freundlich等温吸附模型, 分析结果表明吸附过程可能是易进行的化学吸附. 利用XRD及阴离子交换色谱, 证实了相比于纯黄钾铁矾, 铈的掺杂提高了黄钾铁矾的反应活性, 大幅提升了吸附过程中磷酸根与硫酸根的置换. 通过X射线光电子能谱与红外光谱表征, 推测吸附过程形成稳定的Ce—O—P化学键, 实现特异性化学吸附. 这些研究结果为含磷废水的吸附治理提供了一种新的吸附材料, 并有望为黄钾铁矾的改性和资源化利用提供参考.
刘俊瑞 , 陈晶琳 , 杨杰 , 许肖锋 , 李若男 , 黄有桂 , 陈少华 , 叶欣 , 王维 . 钾位铈掺杂黄钾铁矾的磷吸附特性及机理研究※[J]. 化学学报, 2022 , 80(4) : 476 -484 . DOI: 10.6023/A21120603
Jarosite is a common iron-containing mineral. Researchers have studied its application for removing aqueous pollutants, such as Cr(VI) and As(V). Surprisingly, it shows adsorption for arsenates, but little for the structurally similar phosphate ions. In this study, we prepare cerium doped jarosite and prove the successful doping of cerium at the K+ site by X-ray diffraction (XRD), inductively coupled plasma-optical emission spectrometry (ICP-OES), energy dispersive spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). Phosphorus adsorption experiments show that the small amount of cerium doping (Ce content: 8.75×10-5 mol/g) significantly improves the phosphate adsorption of jarosite, from 1.69 mg/g to 29.33 mg/g (pH=7, 24 h). The phosphate adsorption of Ce-doped jarosite exhibits good pH stability (from pH=3 to pH=11) and excellent selectivity, which is capable of maintaining more than 91% of its adsorption capacity in the presence of various competing anions, such as HCO3-, CO32-, humic acid anion, SO42-, NO3-, and SiO32-. Further analysis reveals that the adsorption process obeys the pseudo-second order kinetic model while the adsorption isotherms represent the Freundlich isotherm. The analysis indicates that the adsorption may be a chemical adsorption process that is easy to proceed. To explore the mechanism of adsorption enhancement, we first characterize the Zeta-potential of the pure jarosite and Ce-doped jarosite. The result indicates similarity of the surface potential between the two samples, which rules out the electrostatic adsorption mechanism. Next, based on the result of anion exchange chromatography, we confirm that the cerium doping greatly increases the exchange between the sulfate groups in jarosite and the phosphate groups in solution, from 2.85 mg/g to 24.90 mg/g. Finally, XPS high-resolution spectroscopy reveals that the chemical environment of Ce changes after the phosphate adsorption, likely indicating the formation of Ce—O—P chemical bonds to achieve specific chemisorption. These results may provide insights for the modification and application of jarosite, as a new adsorbent material for treating phosphorus rich wastewater.

Key words: jarosite; rare-earth doping; adsorption; phosphate pollutant; cerium
| [1] | Strayer, D. L.; Dudgeon, D. J. N. Am. Benthol. Soc. 2010, 29, 344. |
| [2] | Yang, X. H.; Cai, J. B.; Chen, L. H.; Cao, X.; Liu, H. Z.; Liu, M. X.; Chem. Eng. J. 2021, 425, 130623. |
| [3] | Zhang, Z.; Bi, X.; Li, X. T.; Zhao, Q. C.; Chen, H. H. RSC Adv. 2018, 8, 33583. |
| [4] | Eskandarpour, A.; Sassa, K.; Bando, Y.; Okido, M.; Asai, S. Mater. Trans. 2006, 47, 1837. |
| [5] | Wang, Y.; Wang, J. J.; Li, P.; Qin, H. B.; Liang, J. J.; Fan, Q. H. Environ. Technol. Innovation 2021, 23, 101615. |
| [6] | Elwood Madded, M. E.; Bodnar, R. J.; Rimstidt, J. D. Nature 2004, 431, 821. |
| [7] | Dutrizac, J. E.; Jambor, J. L. Rev. Mineral. Geochem. 2000, 40, 405. |
| [8] | Jin, X. H.; Li, X. F.; Guo, C.; Jiang, M. G.; Yao, Q.; Lu, G. N.; Dang, Z. Sci. Total Environ. 2020, 719, 137311. |
| [9] | Karimian, N.; Johnston, S. G.; Burton, E. D. Environ. Sci. Technol. 2017, 51, 4259. |
| [10] | Fan, C.; Guo, C. L.; Zeng, Y. F.; Tu, Z. H.; Ji, Y. P.; Reinfelder, J. R.; Chen, M. Q.; Huang, W. L.; Lu, G. N.; Yi, X. Y.; Dang, Z. Chemosphere 2019, 222, 945. |
| [11] | Tang, Y. G.; Xie, Y. Y.; Lu, G. N.; Ye, H.; Dang, Z.; Wen, Z. N.; Tao, X. Q.; Xie, C. S.; Yi, X. Y. Chemosphere 2020, 255, 126938. |
| [12] | Li, H.; Wang, N. N.; Xiao, T. F.; Zhang, X. T.; Wang, J. Q.; Tang, J. F.; Kong, Q. N.; Fu, C. B.; Quan, H. B. Chemosphere 2021, 285, 131525. |
| [13] | Dutrizac, J. E.; Chen, T. T. Hydrometallurgy 2010, 102, 5. |
| [14] | Mayakaduwage, S.; Mosley, L. M.; Marschner, P. Geoderma 2020, 371, 114359. |
| [15] | Liu, X. W.; Byrne, R. H. Geochim. Cosmochim. Acta 1997, 61, 1625. |
| [16] | Gu, W.; Xie, Q.; Xing, M. C.; Wu, D. Y. Chem. Eng. Res. Des. 2017, 117, 706. |
| [17] | Hassan, M. H.; Stanton, R.; Secora, J.; Trivedi, D. J.; Andreescu, S. ACS Appl. Mater. Interfaces 2020, 12, 52788. |
| [18] | Neale, Z. G.; Barta, M.; Cao, G. Z. ACS Appl. Energ. Mater. 2021, 4, 2248. |
| [19] | Shannon, R. D. Acta Cryst. 1976, 32, 751. |
| [20] | De Simone, C. A.; Mascarenhas, Y. P.; Svisero, D. P. Rev. Bras. Geocienc. 1985, 15, 164. |
| [21] | Spratt, H. J.; Rintoul, L.; Avdeev, M.; Martens, W. N. Am. Mineral. 2013, 98, 1633. |
| [22] | Grohol, D.; Huang, Q. Z.; Toby, B. H.; Lynn, J. W.; Lee, Y. S.; Nocera, D. G. Phys. Rev. B 2003, 68, 94404. |
| [23] | Grohol, D.; Nocera, D. G. J. Am. Chem. Soc. 2002, 124, 2640. |
| [24] | Basciano, L. C. Ph.D. Dissertation, Queen's University, Kingston, 2008. |
| [25] | Labib, S.; Abdelaal, S.; Abdelhady, A. M.; Elmaghraby, E. K. Mater. Chem. Phys. 2020, 256, 123654. |
| [26] | Basciano, L. C.; Peterson, R. C. Am. Mineral. 2008, 93, 853. |
| [27] | Hernández-Lazcano, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Toro, N.; Karthik, T. V. K.; Mendoza-Anaya, D.; Fernández-García, M. E.; Rodríguez-Lugo, V.; Salinas-Rodríguez, E. Minerals 2021, 11, 80. |
| [28] | Zhang, Y.; Xi, X. Q.; Xu, S. N.; Zhou, J. C.; Zhou, J. J.; Xu, Q. H.; Shen, H. Y. Acta Chim. Sinica 2012, 70, 1839. (in Chinese) |
| [28] | (张蕴, 奚晓青, 许姗妮, 周俊晨, 周津金, 徐启宏, 沈昊宇, 化学学报, 2021, 70, 1839.) |
| [29] | Xie, J.; Wang, Z.; Fang, D.; Li, C. J.; Wu, D. Y. J. Colloid Interface Sci. 2014, 423, 13. |
| [30] | Trueman, A. M.; Fitzpatrick, R. W.; Mosley, L. M.; McLaughlin, M. J. Chem. Geol. 2021, 561, 120034. |
| [31] | Xu, R.; Zhang, M. Y.; Mortimer, R. J.; Pan, G. Environ. Sci. Technol. 2017, 51, 3418. |
| [32] | Ho, Y. S. Scientometrics 2004, 59, 171. |
| [33] | Ho, Y. S.; McKay, G. Process Biochem. 1999, 34, 451. |
| [34] | Mohan, D.; Singh, K. P.; Singh, V. K. J. Hazard. Mater. 2006, 135, 280. |
| [35] | Ugurlu, M.; Kula, I.; Hamdi Karaoglu, M.; Arslan, Y. Environ. Prog. Sustainable Energy 2009, 28, 547. |
| [36] | Bai, Z. A.; Chen, R. X.; Pang, H. W.; Wang, X. X.; Song, G.; Yu, S. J. Acta Chim. Sinica 2021, 79, 1265. (in Chinese) |
| [36] | (白子昂, 陈瑞兴, 庞宏伟, 王祥学, 宋刚, 于淑君, 化学学报, 2021, 79, 1265.) |
| [37] | Yang, A. L. Rare Met. Mater. Eng. 2018, 47, 1583. (in Chinese) |
| [37] | (杨爱丽, 稀有金属材料, 2018, 47, 1583.) |
| [38] | Haghseresht, F.; Lu, G. Q. Energ Fuels 1998, 12, 1100. |
| [39] | Wang, G. Z.; Zeng, W.; Li, S. S. Environ. Sci. 2021, 42, 4815. (in Chinese) |
| [39] | (王光泽, 曾薇, 李帅帅, 环境科学, 2021, 42, 4815.) |
| [40] | Wang, F.; Liao, Q. L.; Chen, K. R.; Pan, S. Q.; Lu, M. W. J. Non-Cryst. Solids 2015, 409, 76. |
| [41] | Pemba-Mabiala, J. M.; Lenzi, M.; Lenzi, J.; Lebugle, A. Surf. Interface Anal. 1990, 15, 633. |
| [42] | Mao, D. S.; Luo, Z. H.; Li, Z. Y.; Hong, L.; Qu, R. F.; Wang, J. Q.; Jiang, L. J. Mol. Catal. (China) 2018, 32, 315. (in Chinese) |
| [42] | (冒德寿, 罗子豪, 李智宇, 洪鎏, 曲荣芬, 王家强, 姜亮, 分子催化, 2018, 32, 315.) |
| [43] | Kim, Y. J.; Wolf, A. S.; Becker, U. Geochim. Cosmochim. Acta 2019, 248, 138. |
| [44] | Qi, P. F.; Pichler, T. J. Hazard. Mater. 2017, 330, 142. |
| [45] | Chen, K.; Jin, X. H.; Guo, C. L.; He, C. C.; Zhang, Y. Y.; Gao, K.; Lu, G. N.; Dang, Z. Chem. Geol. 2021, 579, 120338. |
| [46] | Derycke, V.; Kongolo, M.; Benzaazoua, M.; Mallet, M.; Barrès, O.; De Donato, P.; Bussière, B.; Mermillod-Blondin, R. Int. J. Miner. Process. 2013, 118, 1. |
| [47] | Neal, A. L.; Techkarnjanaruk, S.; Dohnalkova, A.; Mccready, D.; Peyton, B. M.; Geesey, G. G. Geochim. Cosmochim. Acta 2001, 65, 223. |
| [48] | Siriwardane, R. V.; Cook, J. M. J. Colloid Interface Sci. 1986, 114, 525. |
| [49] | Cao, L. N.; Chen, B. H.; Gou, X. Y.; Zou, Q. Geol. J. Chin. Univ. 2019, 25, 333. (in Chinese) |
| [49] | (曹丽娜, 陈炳辉, 苟习颖, 邹琦, 高等地质学报, 2019, 25, 333.) |
| [50] | Bi, S. F.; Cui, X. M. J. Salt. Chem. Ind. 2015, 44, 26. (in Chinese) |
| [50] | (毕思峰, 崔香梅, 盐业与化工, 2015, 44, 26.) |
/
| 〈 |
|
〉 |