

HMOR分子筛骨架铝分布研究及二甲醚羰基化反应活性中心的辨识※
收稿日期: 2022-01-09
网络出版日期: 2022-03-11
基金资助
国家自然科学基金(21972142); 国家自然科学基金(22022202); 国家自然科学基金(21991092); 国家自然科学基金(21991090); 大连市杰出青年基金项目(2021RJ01); 中国科学院前沿科学重点研究计划(QYZDY-SSW-JSC024); 中国科学院国际合作(121421KYSB20180007)
Study on the Framework Aluminum Distributions of HMOR Zeolite and Identification of Active Sites for Dimethyl Ether Carbonylation Reaction※
Received date: 2022-01-09
Online published: 2022-03-11
Supported by
National Natural Science Foundation of China(21972142); National Natural Science Foundation of China(22022202); National Natural Science Foundation of China(21991092); National Natural Science Foundation of China(21991090); Dalian Outstanding Young Scientist Foundation(2021RJ01); Key Research Program of Frontier Sciences, Chinese Academy of Sciences(QYZDY-SSW-JSC024); International Partnership Program of Chinese Academy of Sciences(121421KYSB20180007)
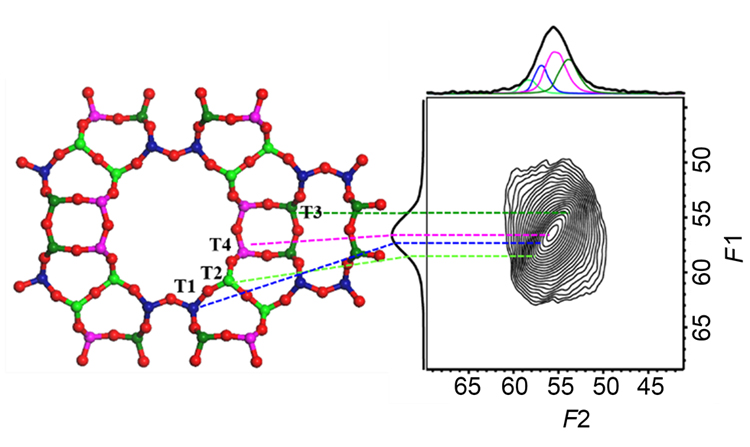
HMOR分子筛在二甲醚羰基化反应中具有类似酶催化的优异性能. 关于骨架铝的分布和反应活性位的识别是研究该反应机理的关键科学问题. 早期的工作是基于理论计算研究二甲醚羰基化活性位点, 但缺乏直接的谱学证据. 通过在不同温度下焙烧NH4MOR制备了一系列HMOR催化剂, 通过多种谱学表征手段研究分子筛骨架铝的稳定性以及铝原子落位信息, 进一步通过二甲醚羰基化反应活性关联MOR分子筛的酸性和铝分布关系获得反应机理的谱学证据. 首先从XRD (X-Ray diffraction)和SEM (Scanning electron microscope)发现经过不同温度焙烧, MOR分子筛结晶度和宏观形貌没有发生明显变化, 但是通过一维29Si, 27Al和1H魔角旋转固体核磁谱(MAS NMR)发现分子筛局部环境发生了脱铝现象, 产生了明显的缺陷羟基以及B酸量的下降. 焙烧温度对HMOR分子筛骨架Al稳定性的影响较大, 随着温度升高, 脱铝逐渐加剧. 定量1H MAS NMR结合红外(IR)光谱提供了HMOR分子筛不同孔道B酸含量的分布. 进一步使用2D 27Al MQ MAS NMR的方法以及结合切片分峰拟合技术区分出分子筛骨架中的四种不同T位点, 发现当温度低于600 ℃, 不同T位脱铝速率相当; 当焙烧温度为600 ℃时, T3位点的Al原子脱除速率加快. 最后研究了二甲醚羰基化反应性能与酸分布和铝分布的关系, 获得羰基化反应活性中心的确凿谱学证据, T3-O33位置的Al位是羰基化反应的活性中心.
张瑾 , 丁湘浓 , 刘红超 , 樊栋 , 徐舒涛 , 魏迎旭 , 刘中民 . HMOR分子筛骨架铝分布研究及二甲醚羰基化反应活性中心的辨识※[J]. 化学学报, 2022 , 80(5) : 590 -597 . DOI: 10.6023/A22010014
HMOR zeolites has an excellent performance similar to enzyme catalysis in the carbonylation of dimethyl ether (DME). The distribution of framework aluminum and the identification of the active site of the reaction are the key issues in the study of the reaction mechanism. The early work was based on theoretical calculation to study the active site of DME carbonylation, but lacked direct experimental evidence. In this work, a series of HMOR catalysts were prepared by calcination of NH4MOR at various temperatures. The stability and location of framework aluminum were studied by a variety of spectroscopic characterization methods. Moreover, the evidence of reaction mechanism was obtained by the carbonylation reaction activity of dimethyl ether related to the acidity of MOR zeolite and aluminum distribution. Firstly, it was found that the crystallinity and morphology of MOR zeolites did not change significantly after calcination at different temperatures by XRD (X-Ray diffraction) and SEM (Scanning electron microscope). However, it was found by 29Si, 27Al and 1H Magic angle spinning (MAS) solid-state nuclear magnetic resonance (NMR) that the local environment of HMORs was dealuminated, which resulted in obvious defect hydroxyl groups and the decrease of Brönsted acid sites (BASs) content. In addition, the calcination temperature has a great influence on the stability of framework Al of HMORs. Increase of calcination temperature will accelerate the occurrence of dealumination. Quantitative 1H MAS NMR combined with Fourier transform infrared spectra (FTIR) provided the distribution of BASs content in different channels of HMOR zeolites. By using 2D 27Al multiple quantum (MQ) MAS NMR method combined with the representative slices parallel to the F2 dimension of MQMAS NMR spectra at selected F1 chemical shift to distinguish the framework Al sites, it was found that when the temperature was lower than 600 ℃, framework Al atoms located in the different T-sites had the similar dealumination rate. But when the calcination temperature was increased to 600 ℃, the removal rate of Al atom at T3 site was accelerated. Furthermore, the relationship between the carbonylation performance of dimethyl ether and the distribution of Brønsted acid and aluminum was studied, and the definitive spectral evidence of the carbonylation activity center was obtained, that is, the Al site at T3-O33 was the active site of the carbonylation reaction.

Key words: HMOR; acidity; aluminum distribution; solid-state NMR; DME carbonylation
| [1] | Sun, D.; Sun, B.; Pei, Y.; Yan, S. R.; Fan, K. N.; Qiao, M. H.; Zhang, X. X.; Zong, B. N. Acta Chim. Sinica 2021, 79, 771. (in Chinese) |
| [1] | (孙冬, 孙博, 裴燕, 闫世润, 范康年, 乔明华, 张晓昕, 宗保宁, 化学学报, 2021, 79, 771.) |
| [2] | Zhang, M. T.; Yan, T. T.; Dai, W. L.; Guan, N. J.; Li, L. D. Acta Chim. Sinica 2020, 78, 1404. (in Chinese) |
| [2] | (张梦婷, 颜婷婷, 戴卫理, 关乃佳, 李兰冬, 化学学报, 2020, 78, 1404.) |
| [3] | Yao, X. T.; Huang, X.; Lin, Y. X.; Liu, Y. M. Acta Chim. Sinica 2020, 78, 1111. (in Chinese) |
| [3] | (姚旭婷, 黄鑫, 林玉霞, 刘月明, 化学学报, 2020, 78, 1111.) |
| [4] | Liu, Y. H.; Zhao, N.; Xian, H.; Cheng, Q. P.; Tan, Y. S.; Tsubaki, N.; Li, X. G. ACS Appl. Mater. Inter. 2015, 7, 8398. |
| [5] | Li, Y.; Sun, Q.; Huang, S. Y.; Cheng, Z. Z.; Cai, K.; Lv, J.; Ma, X. B. Catal. Today 2018, 311, 81. |
| [6] | Zhao, N.; Cheng, Q. P.; Lyu, S. S.; Guo, L. H.; Tian, Y.; Ding, T.; Xu, J.; Ma, X. B.; Li, X. G. Catal. Today. 2020, 339, 86. |
| [7] | Huo, H.; Peng, L. M.; Gan, Z. H.; Grey, C. P. J. Am. Chem. Soc. 2012, 134, 9708. |
| [8] | Lukyanov, D. B.; Vazhnova, T.; Cherkasov, N.; Casci, J. L.; Birtill, J. J. J. Phys. Chem. C 2014, 118, 23918. |
| [9] | Cai, K.; Huang, S. Y.; Li, Y.; Cheng, Z. Z.; Lv, J.; Ma, X. B. ACS Sustain. Chem. Eng. 2019, 7, 2027. |
| [10] | Yi, X. F.; Xiao, Y.; Li, G. C.; Liu, Z. Q.; Chen, W.; Liu, S. B.; Zheng, A. M. Chem. Mater. 2020, 32, 1332. |
| [11] | Gong, K.; Liu, Z. M.; Liang, L. X.; Zhao, Z. C.; Guo, M. L.; Liu, X. B.; Han, X. W.; Bao, X. H.; Hou, G. J. J. Phys. Chem. Lett. 2021, 12, 2413. |
| [12] | Cao, K. P.; Fan, D.; Gao, M. B.; Fan, B. H.; Chen, N.; Wang, L. Y.; Tian, P.; Liu, Z. M. ACS Catal. 2021, 12, 1. |
| [13] | Bajpai, P. K. Zeolites 1986, 6, 2. |
| [14] | Bajpai, P. K.; Rao, M. S.; Gokhale, K. V. G. K. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 721. |
| [15] | Bhan, A.; Allian, A. D.; Sunley, G. J.; Law, D. J.; Iglesia, E. J. Am. Chem. Soc. 2007, 129, 4919. |
| [16] | Boronat, M.; Martinez-Sanchez, C.; Law, D.; Corma, A. J. Am. Chem. Soc. 2008, 130, 16316. |
| [17] | Cheung, P.; Bhan, A.; Sunley, G. J.; Iglesia, E. Angew. Chem., Int. Ed. 2006, 45, 1617. |
| [18] | Cheung, P.; Bhan, A.; Sunley, G.; Law, D.; Iglesia, E. J. Catal. 2007, 245, 110. |
| [19] | Jiang, Y. J.; Hunger, M.; Wang, W. J. Am. Chem. Soc. 2006, 128, 11679. |
| [20] | Chu, Y. Y.; Lo, A. Y.; Wang, C.; Deng, F. J. Phys. Chem. C 2019, 123, 15503. |
| [21] | Blasco, T.; Boronat, M.; Concepcion, P.; Corma, A.; Law, D.; Vidal-Moya, J. A. Angew. Chem., Int. Ed. 2007, 46, 3938. |
| [22] | He, T.; Ren, P. J.; Liu, X. C.; Xu, S. T.; Han, X. W.; Bao, X. H. Chem. Commun. 2015, 51, 16868. |
| [23] | Li, B. J.; Xu, J.; Han, B.; Wang, X. M.; Qi, G. D.; Zhang, Z. F.; Wang, C.; Deng, F. J. Phys. Chem. C 2013, 117, 5840. |
| [24] | Xue, H. F.; Huang, X. M.; Ditzel, E.; Zhan, E. S.; Ma, M.; Shen, W. J. Ind. Eng. Chem. Res. 2013, 52, 11510. |
| [25] | Wang, S. R.; Guo, W. W.; Zhu, L. J.; Wang, H. X.; Qiu, K. Z.; Cen, K. F. J. Phys. Chem. C 2014, 119, 524. |
| [26] | Liu, S. P.; Liu, H. C.; Ma, X. G.; Liu, Y.; Zhu, W. L.; Liu, Z. M. Catal. Sci. Technol. 2020, 10, 4663. |
| [27] | Cao, K. P.; Fan, D.; Li, L. Y; Fan, B. H.; Wang, L. Y.; Zhu, D. L.; Wang, Q. Y.; Tian, P.; Liu, Z. M. ACS Catal. 2020, 10, 3372. |
| [28] | Wang, X. S.; Li, R. J.; Yu, C. C.; Liu, Y. X.; Zhang, L. Y.; Xu, C. M.; Zhou, H. J. Fuel 2019, 239, 794. |
| [29] | Reule, A. A. C.; Sawada, J. A.; Sema gina, N. J. Catal. 2017, 349, 98. |
| [30] | Li, Y.; Li, Z. H.; Huang, S. Y.; Cai, K.; Qu, Z.; Zhang, J. F.; Wang, Y.; Ma, X. B. ACS Appl. Mater. Inter. 2019, 11, 24000. |
| [31] | Paul, G.; Bisio, C.; Braschi, I.; Cossi, M.; Gatti, G.; Gianotti, E.; Marchese, L. Chem. Soc. Rev. 2018, 47, 5684. |
| [32] | Chen, X.; Fu, Y. Y.; Yue, B.; He, H. Y. Chin. J. Magn. Reson. 2021, 38, 491. (in Chinese) |
| [32] | (陈欣, 付颖懿, 岳斌, 贺鹤勇, 波谱学杂志, 2021, 38, 491.) |
| [33] | Gao, S. S.; Xu, S. T.; Wei, Y. X.; Liu, Z. M. Chin. J. Magn. Reson. 2021, 38, 433. (in Chinese) |
| [33] | (高树树, 徐舒涛, 魏迎旭, 刘中民, 波谱学杂志, 2021, 38, 433.) |
| [34] | Xao, Y.; Xia, C. J.; Yi, X. F.; Liu, F. Q.; Liu, S. B.; Zheng, A. M. Chin. J. Magn. Reson. 2021, 38, 571. (in Chinese) |
| [34] | 肖瑶, 夏长久, 易先锋, 刘凤庆, 刘尚斌, 郑安民, 波谱学杂志, 2021, 38, 571.) |
| [35] | Chen, H. D.; Kong, H. Y.; Zhao, Z. C.; Zhang, W. P. Chin. J. Magn. Reson. 2021, 38, 543. (in Chinese) |
| [35] | (陈翰迪, 孔海宇, 赵侦超, 张维萍, 波谱学杂志, 2021, 38, 543.) |
| [36] | Yang, W. J.; Huang, J. Chin. J. Magn. Reson. 2021, 38, 460. (in Chinese) |
| [36] | (杨文杰, 黄骏, 波谱学杂志, 2021, 38, 460.) |
| [37] | Wang, Y. X. Wang, Q.; Xu, J.; Xia, Q. H.; Deng, F. Chin. J. Magn. Reson. 2021, 38, 514. (in Chinese) |
| [37] | (王永祥, 王强, 徐君, 夏清华, 邓风, 波谱学杂志, 2021, 38, 514.) |
| [38] | Xia, X. F.; Zhang, W. J.; Lin, Z. Y.; Ke, X. K.; Wen, Y. J.; Wang, F.; Chen, J. C.; Peng, L. M. Chin. J. Magn. Reson. 2021, 38, 533. (in Chinese) |
| [38] | (夏锡锋, 张文静, 林芝晔, 柯晓康, 温玉洁, 王芳, 陈俊超, 彭路明, 波谱学杂志, 2021, 38, 533.) |
| [39] | Fyfe, C. A.; Gobbi, G. C.; Murphy, W. J.; Ozubko, R. S.; Slack, D. A. J. Am. Chem. Soc. 1984, 106, 4435. |
| [40] | Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J. N.; Tatsumi, T. J. Phys. Chem. C 2015, 119, 15303. |
| [41] | Kneller, J. M.; Pietraß, T.; Ott, K. C.; Labouriau, A. Micropor. Mesopor. Mater. 2003, 62, 121. |
| [42] | Ravi, M.; Sushkevich, V. L.; van Bokhoven, J. A. Chem. Sci. 2021, 12, 4094. |
| [43] | Medek, A.; Harwood, J. S.; Frydman, L. J. Am. Chem. Soc. 1995, 117, 12779. |
| [44] | Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z. H.; Hoatson, G. Magn. Reason. Chem. 2002, 40, 70. |
| [45] | Hu, J. Z.; Wan, C.; Vjunov, A.; Wang, M.; Zhao, Z. C.; Hu, M. Y.; Camaioni, D. M.; Lercher, J. A. J. Phys. Chem. C. 2017, 121, 12849. |
| [46] | Frydman, L.; Harwood, J. S. J. Am. Chem. Soc. 1995, 117, 5367. |
| [47] | Sarv, P.; Tuherm, T.; Lippmaa, E.; Keskinen, K.; Root, A. J. Phys. Chem. 1995, 99, 13763. |
| [48] | Maache, M.; Janin, A.; Lavalley, J. C.; Benazzi, E. Zeolites 1995, 15, 507. |
| [49] | Pastvova, J.; Pilar, R.; Moravkova, J.; Kaucky, D.; Rathousky, J.; Sklenak, S.; Sazama, P. Appl. Catal. A. 2018, 562, 159. |
| [50] | Wakabayashi, F.; Kondo, J.; Wada, A.; Domen, K.; Hirose, C. J. Phys. Chem. 1996, 100, 4154. |
| [51] | Zholobenko, V. L.; Makarova, M. A.; Dwyer, J. J. Phys. Chem. 2002, 97, 5962. |
| [52] | Engelhardt, G.; Kentgens, A. P. M.; Koller, H.; Samoson, A. Solid State Nucl. Magn. Reson. 1999, 15, 171. |
| [53] | Liu, R. S.; Fan, B. H.; Zhang, W. N.; Wang, Y. L.; Qi, L.; Xu, S. T.; Yu, Z. X.; Wei, Y. X.; Liu, Z. M. Angew. Chem., Int. Ed. 2022, e202116990. |
| [54] | Jeffroy, M.; Nieto-Draghi, C.; Boutin, A. Chem. Mater. 2017, 29, 513. |
| [55] | Chen, K.; Horstmeier, S.; Nguyen, V. T.; Wang, B.; Crossley, S. P.; Pham, T.; Gan, Z.; Hung, I.; White, J. L. J. Am. Chem. Soc. 2020, 142, 7514. |
| [56] | Chen, K.; Gan, Z.; Horstmeier, S.; White, J. L. J. Am. Chem. Soc. 2021, 143, 6669. |
| [57] | Bodart, P.; Nagy, J. B.; Debras, G.; Gabelica, Z.; Jacobs, P. A. J. Phys. Chem. 1986, 90, 5183. |
| [58] | Debras, G.; Nagy, J. B.; Gabelica, Z.; Bodart, P.; Jacobs, P. A. Chem. Lett. 1983, 12, 199. |
| [59] | Schroeder, K. P.; Sauer, J. J. Phys. Chem. 1993, 97, 6579. |
| [60] | Amoureux, J. P.; Fernandez, C.; Steuernagel, S. J. Magn. Reson. A 1996, 123, 116. |
/
| 〈 |
|
〉 |