

N^C^N型Pt(II)配合物与大环主体葫芦[10]脲的识别及发光性质研究
收稿日期: 2022-02-20
网络出版日期: 2022-04-01
基金资助
国家自然科学基金(21871216); 国家自然科学基金(21901194)
Recognition and Luminescence Properties of N^C^N Pt(II) Complexes with Macrocyclic Host Cucurbit[10]uril
Received date: 2022-02-20
Online published: 2022-04-01
Supported by
National Natural Science Foundation of China(21871216); National Natural Science Foundation of China(21901194)
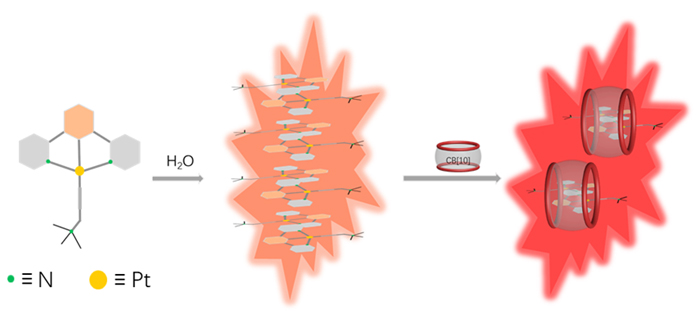
由于过渡金属配合物具有独特的光物理化学性质而被广泛研究. 其中Pt(Ⅱ)配合物发生组装时会因Pt(Ⅱ)-Pt(Ⅱ)之间的距离不同而显示不同的荧光特性, 而主客体相互作用可以影响发光小分子的排列及组装. 为进一步探究主客体相互作用对Pt(Ⅱ)配合物发光性能的影响, 设计合成了不同取代的N^C^N型Pt(Ⅱ)配合物, 研究了大环主体葫芦[10]脲(CB[10])对这类配合物的识别作用及包合物的光谱性质. 核磁共振氢谱和质谱证明CB[10]可与配合物以1∶2的比例结合. 紫外-可见吸收光谱和荧光发射光谱分析表明主客体作用对该类金属配合物光谱性质有较大影响, 所形成的主客体包合物的磷光寿命及量子产率都有不同程度的变化. 研究结果表明, CB[10]可通过包结两个Pt(Ⅱ)配合物分子, 拉近铂原子之间的距离, 增强该类配合物在水相中的Pt(II)…Pt(II)相互作用和π-π相互作用, 实现水相中的长寿命磷光发射. 同时, 主客体作用对这类金属配合物的力致变色性质也有一定的影响.
关键词: 金属配合物; N^C^N配体; Pt(Ⅱ)-Pt(Ⅱ)相互作用; 主客体相互作用; 葫芦[10]脲
朱诗敏 , 黄鑫 , 韩勰 , 刘思敏 . N^C^N型Pt(II)配合物与大环主体葫芦[10]脲的识别及发光性质研究[J]. 化学学报, 2022 , 80(8) : 1066 -1070 . DOI: 10.6023/A22020078
Transition metal complexes have been widely studied due to their unique photophysical and chemical properties. In order to further explore the influence of host-guest interaction on luminescent property of Pt complexes, three different substituted water-soluble Pt complexes were designed and synthesized. Cucurbit[10]uril (CB[10]), with the largest cavity among the cucurbit[n]uril family, was selected as the host molecule. The 1H NMR titration experiments proved CB[10] binded three guests with 1∶2 stoichiometry, and the 1,3-di(2-pyridyl)benzene (N^C^N) ligand part on the guest molecule was encapsulated antiparallelly in the cavity of CB[10] in a head-to-tail manner. Mass spectrometry characterization further proved the binding between host and guest with 1∶2 stoichiometry. The low-energy absorption peaks of N^C^N Pt(II) complexes at >400 nm attributed to metal-metal-to-ligand charge transfer (MMLCT) were enhanced upon binding with CB[10]. Compared to free [2,6-di(2-pyridinyl)phenyl][3-(trimethylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-1), the 3IL emission intensity of its complex with CB[10] was enhanced dominantly. The [methyl 3,5-di(2-pyridyl)benzoato][3-(tri- methylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-2) showed visible red phosphorescence emission at 648 nm which was attributed to 3MMLCT in aqueous solution. Interestingly, binding with CB[10] led to a red-shift to 667 nm, which was closer to near-infrared emission. Besides, the phosphorescence lifetime is enhanced by 1.5 times (14.64 to 23.74 μs), and the quantum yield is enhanced by 15.6 times (2.7% to 42%) upon the host-guest complexation. The luminescent property of [4-methoxy-2,6-di(2-pyridinyl)phenyl][3-(trimethylammonio)-1-propyn-1-yl]platinum(II) chloride (Pt-3) was similar to that of Pt-2. Further research on the luminescence changes of Pt complexes before and after grinding in solid state indicated that the host-guest complex showed bigger red-shift of emission wavelength than free guest. The above results showed that the host-guest interactions based on CB[10] could shorten the distance between the platinum atoms of the two included guest molecules, thus enhancing metal-metal interaction and π-π interaction of the host-guest complexes.

| [1] | (a) Wong, K. M.-C.; Yam, V. W.-W. Acc. Chem. Res. 2011, 44, 424. |
| [1] | (b) Lai, P.-N.; Brysacz, C. H.; Alam, M. K.; Ayoub, N. A.; Gray, T. G.; Bao, J.; Teets, T. S. J. Am. Chem. Soc. 2018, 140, 10198. |
| [2] | (a) Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N. S.; De Cola, L. Chem. Soc. Rev. 2014, 43, 4144. |
| [2] | (b) Chan, A. K.-W.; Ng, M.; Wong, Y.-C.; Chan, M.-Y.; Wong, W.-T.; Yam, V. W.-W. J. Am. Chem. Soc. 2017, 139, 10750. |
| [2] | (c) Lin, J.; Zou, C.; Zhang, X.; Gao, Q.; Suo, S.; Zhuo, Q.; Chang, X.; Xie, M.; Lu, W. Dalton Trans. 2019, 48, 10417. |
| [2] | (d) Allampally, N. K.; Bredol, M.; Strassert, C. A.; De Cola, L. Chem.-Eur. J. 2014, 20, 16863. |
| [3] | Li, K.; Ming Tong, G. S.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Chem. Sci. 2016, 7, 1653. |
| [4] | Chow, P.-K.; To, W.-P.; Low, K.-H.; Che, C.-M. Chem.-Asian J. 2014, 9, 534. |
| [5] | Wang, D.; Chen, Y.; Liu, X.; Bian, J.; Yin, X.; Teng, M.; Rong, M.; Wang, Z. Chin. J. Inorg. Chem. 2021, 37, 33. (in Chinese) |
| [5] | (王登强, 陈宇, 刘小庆, 卞健健, 尹新颖, 滕明瑜, 戎梅竹, 汪正良, 无机化学学报, 2021, 37, 33.) |
| [6] | Xu, G; Li, J.; Chen, Z. Acta Chim. Sinica 2014, 72, 667. (in Chinese) |
| [6] | (徐广涛, 李佳, 陈忠宁, 化学学报, 2014, 72, 667.) |
| [7] | Zhang, S.; Wang, S.; Zhou, X.; Zhang, H.; Shao, S.; Li, C. Acta Chim. Sinica 2008, 66, 841. (in Chinese) |
| [7] | (张首才, 王嵩, 周欣, 张红星, 邵琛, 李传碧, 化学学报, 2008, 66, 841.) |
| [8] | (a) Sun, C.-Y.; To, W.-P.; Hung, F.-F.; Wang, X.-L.; Su, Z.-M.; Che, C.-M. Chem. Sci. 2018, 9, 2357. |
| [8] | (b) Li, Z.; Han, Y.; Gao, Z.; Wang, F. ACS Catal. 2017, 7, 4676. |
| [9] | (a) Baggaley, E.; Botchway, S. W.; Haycock, J. W.; Morris, H.; Sazanovich, I. V.; Williams, J. A. G.; Weinstein, J. A. Chem. Sci. 2014, 5, 879. |
| [9] | (b) Ouyang, C.; Li, Y.; Rees, T. W.; Liao, X.; Jia, J.; Chen, Y.; Zhang, X.; Ji, L.; Chao, H. Angew. Chem., nt. Ed. 2021, 60, 4150. |
| [9] | (c) Law, A. S.-Y.; Lee, L. C.-C.; Lo, K. K.-W.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 5396. |
| [10] | (a) Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Inorg. Chem. 2021, 60, 10350. |
| [10] | (b) Ramu, V.; Gautam, S.; Garai, A.; Kondaiah, P.; Chakravarty, A. R. Inorg. Chem. 2018, 57, 1717. |
| [11] | (a) Summa, G. M.; Scott, B. A. Inorg. Chem. 1980, 19, 1079. |
| [11] | (b) Yip, H.-K.; Cheng, L.-K.; Cheung, K.-K.; Che, C.-M. J. Chem. Soc., Dalton Trans. 1993, 2933. |
| [12] | (a) Yam, V. W.-W.; Tang, R. P.-L.; Wong, K. M.-C.; Cheung, K.-K. Organometallics 2001, 20, 4476. |
| [12] | (b) Du, P.; Schneider, J.; Jarosz, P.; Eisenberg, R. J. Am. Chem. Soc. 2006, 128, 7726. |
| [12] | (c) Yang, Z.; Tian, Y.; Li, Z.; Ao, L.; Gao, Z.; Wang, F. Acta Polym. Sin. 2017, 48, 121. (in Chinese) |
| [12] | (杨支帅, 田玉奎, 李子健, 敖雷, 高宗春, 汪峰, 高分子学报, 2017, 48, 121.) |
| [13] | (a) Yam, V. W.-W.; Wong, K. M.-C.; Zhu, N. J. Am. Chem. Soc. 2002, 124, 6506. |
| [13] | (b) Yam, V. W.-W.; Chan, K. H.-Y.; Wong, K. M.-C.; Zhu, N. Chem.-Eur. J. 2005, 11, 4535. |
| [14] | Williams, J. A. G. Chem. Soc. Rev. 2009, 38, 1783. |
| [15] | (a) Hu, S.-J.; Guo, X.-Q.; Zhou, L.-P.; Yan, D.-N.; Cheng, P.-M.; Cai, L.-X.; Li, X.-Z.; Sun, Q.-F. J. Am. Chem. Soc. 2022, 144, 4244. |
| [15] | (b) Gemen, J.; Ahrens, J.; Shimon, L. J. W.; Klajn, R. J. Am. Chem. Soc. 2020, 142, 17721. |
| [15] | (c) Benson, C. R.; Kacenauskaite, L.; VanDenburgh, K. L.; Zhao, W.; Qiao, B.; Sadhukhan, T.; Pink, M.; Chen, J.; Borgi, S.; Chen, C.-H.; Davis, B. J.; Simon, Y. C.; Raghavachari, K.; Laursen, B. W.; Flood, A. H. Chem 2020, 6, 1978. |
| [16] | (a) Liu, S.; Zavalij, P. Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 127, 16798. |
| [16] | (b) Yang, X.; Liu, F.; Zhao, Z.; Liang, F.; Zhang, H.; Liu, S. Chin. Chem. Lett. 2018, 29, 1560. |
| [16] | (c) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese) |
| [16] | (田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.) |
| [17] | (a) Anis-Ul-Haque, K. M.; Woodward, C. E.; Day, A. I.; Wallace, L. Inorg. Chem. 2020, 59, 3942. |
| [17] | (b) Luis, E. T.; Day, A. I.; König, B.; Beves, J. E. Inorg. Chem. 2020, 59, 9135. |
| [17] | (c) Zhang, Y.; Liu, M.; Karatchevtseva, I.; Price, J. R.; Tao, Z.; Wei, G. New J. Chem. 2020, 44, 18208. |
| [18] | (a) Kuang, S.; Hu, Z.; Zhang, H.; Zhang, X.; Liang, F.; Zhao, Z.; Liu, S. Chem. Commun. 2018, 54, 2169. |
| [18] | (b) Deng, Y.; Yin, H.; Zhao, Z.; Wang, R.; Liu, S. Supramol. Chem. 2018, 30, 706. |
| [19] | Hu, Z.; Sun, D.; Han, X.; Liu, S. Chin. J. Org. Chem. 2020, 40, 1361. (in Chinese) |
| [19] | (胡智雄, 孙冬冬, 韩勰, 刘思敏, 有机化学, 2020, 40, 1361.) |
| [20] | (a) Chen, Y.; Li, K.; Lu, W.; Chui, S. S.-Y.; Ma, C.-W.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 9909. |
| [20] | (b) Williams, J. A. G.; Beeby, A.; Davies, E. S.; Weinstein, J. A.; Wilson, C. Inorg. Chem. 2003, 42, 8609. |
| [20] | (c) Cárdenas, D. J.; Echavarren, A. M.; Ramírez de Arellano, M. C. Organometallics 1999, 18, 3337. |
| [20] | (d) Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Inorg. Chem. 2010, 49, 11276. |
| [21] | Wan, Q.; Xiao, X.-S.; To, W.-P.; Lu, W.; Chen, Y.; Low, K.-H.; Che, C.-M. Angew. Chem., nt. Ed. 2018, 57, 17189. |
| [22] | (a) Chen, Y.; Lu, W.; Che, C.-M. Organometallics 2013, 32, 350. |
| [22] | (b) Ai, Y.; Chan, M. H.-Y.; Chan, A. K.-W.; Ng, M.; Li, Y.; Yam, V. W.-W. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 13856. |
| [23] | Li, B.; Li, Y.; Chan, M. H.-Y.; Yam, V. W.-W. J. Am. Chem. Soc. 2021, 143, 21676. |
| [24] | Xie, M.; Lu, W. Dalton Trans. 2019, 48, 1275. |
| [25] | Zhu, S.; Hu, J.; Zhai, S.; Wang, Y.; Xu, Z.; Liu, R.; Zhu, H. Inorg. Chem. Front. 2020, 7, 4677. |
| [26] | Han, X.; Sun, D.; Tang, S.; Wu, Y.; Wang, L.; Zhang, X.; Liu, S. J. Mater. Chem. C 2021, 9, 17307. |
| [27] | (a) Kozhevnikov, V. N.; Donnio, B.; Bruce, D. W. Angew. Chem., nt. Ed. 2008, 47, 6286. |
| [27] | (b) Choi, S. J.; Kuwabara, J.; Nishimura, Y.; Arai, T.; Kanbara, T. Chem. Lett. 2011, 41, 65. |
| [27] | (c) Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Dalton Trans. 2009, 711. |
| [27] | (d) Zhang, X.-P.; Mei, J.-F.; Lai, J.-C.; Li, C.-H.; You, X.-Z. J. Mater. Chem. C 2015, 3, 2350. |
/
| 〈 |
|
〉 |