

聚乙烯醇调控水溶性共轭聚噻吩的光学性质
收稿日期: 2022-01-08
网络出版日期: 2022-04-06
基金资助
国家重点研发计划国际合作项目(2020YFE0100300); 国家自然科学基金(22072036); 国家自然科学基金(21905072); 国家自然科学基金(22122206); 天津市自然科学基金(20JCQNJC00890); 河北省自然科学基金(B2020202034); 河北省自然科学基金(B2020202086); 河北省高等学校科学技术研究(BJ2020039)
Regulating Optical Properties of Water-Soluble Conjugated Polythiophene with Polyvinyl Alcohol
Received date: 2022-01-08
Online published: 2022-04-06
Supported by
National Key Research and Development Program of China(2020YFE0100300); National Natural Science Foundation of China(22072036); National Natural Science Foundation of China(21905072); National Natural Science Foundation of China(22122206); Natural Science Foundation of Tianjin(20JCQNJC00890); Natural Science Foundation of Hebei Province(B2020202034); Natural Science Foundation of Hebei Province(B2020202086); Science and Technology Research Project of Higher Education in Hebei Province(BJ2020039)
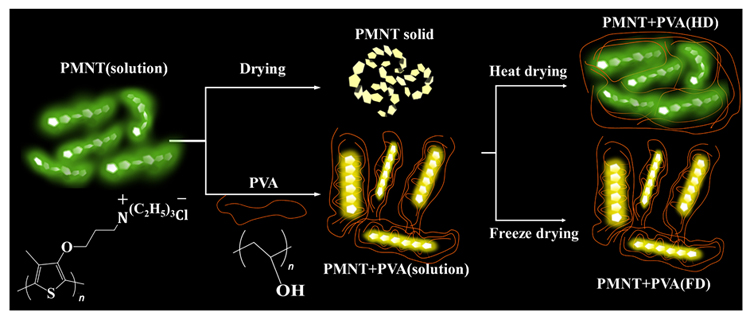
针对发光共轭聚合物稀溶液在干燥形成固体时的荧光淬灭问题, 通过高分子聚乙烯醇(PVA)的氢键网络调控水溶性共轭聚噻吩在溶液中的聚集行为和构象, 并采用不同的干燥方式实现了调控其固体光学性质的目的. 紫外-可见光吸收、荧光发射光谱测试表明, 在水溶液中PVA可以分散共轭聚合物链, 并增强其共平面性; 高温干燥后, 聚噻吩薄膜与无PVA添加的聚噻吩溶液的荧光性质相似; 而采用冷冻干燥法, 薄膜则保留了添加PVA后混合溶液的发光特性. 该结果表明, PVA对聚噻吩在溶液状态下的聚集/分子构象的调控行为随干燥方式的不同得到了不同程度的保留——高温加热干燥仅维持了PVA对聚噻吩的分散作用; 而冷冻干燥则完整保留了PVA与聚噻吩的分子间相互作用, 将溶液中分子的分散状态和构象同时固定. 本研究从干燥方式的角度为固态共轭聚合物聚集行为及发光性质的调控提供了新的策略.
齐子朋 , 高冬 , 朱志成 , 贺志远 , 白国英 . 聚乙烯醇调控水溶性共轭聚噻吩的光学性质[J]. 化学学报, 2022 , 80(7) : 921 -928 . DOI: 10.6023/A22010013
Water-soluble conjugated polymers have attracted enormous attention because of their water solubility and attractive optical properties. However, they often suffer from low emission quantum yield due to their strong inter-chain interaction and concomitant exciton quenching, especially in the solid state. To enhance the emission of the water-soluble conjugated polymers, strategies of decreasing the aggregation tendency of the polymers and controlling the backbone conformations are often adopted. In this work, to solve the problem of aggregation-caused quenching of luminescent conjugated polymer dilute solution during drying, we regulate the aggregation behavior and backbone conformation of a water-soluble conjugated polythiophene (PMNT) with polyvinyl alcohol (PVA) by intermolecular interactions, e.g. hydrogen bonding, and obtained tunable emissive PMNT thin films. Specifically, when PMNT in solution state is blended with PVA, obvious red shift of absorption and emission maxima and higher fluorescence intensity are observed, indicating that PVA molecules isolate the PMNT chains, reduce the degree of chain flexibility and increase backbone rigidity. Furthermore, by varying dry methods, we obtained PMNT solid films of tunable light absorption/emission properties: the solid films fabricated through splat cooling and in-situ lyophilization preserve the optical properties of the mixed solution of PVA and PMNT; the films fabricated through heat drying exhibit the similar optical properties with those of PMNT solution without adding PVA. Moreover, the emission quantum yield of the solid film prepared by the lyophilization method was enhanced compared with that of the solid film prepared by heat drying. These results suggest that the aggregation/conformation regulations of PMNT solution by PVA are retained in different degrees dependent on the drying methods: heat drying only maintains the isolation effect of PVA on PMNT; lyophilization readily preserve the interactions between PVA and PMNT, locking their disperse states and conformations. This study provides a strategy for regulating the aggregation behaviors and optical properties of solid conjugated polymers from the perspective of drying method.

| [1] | Feng, X. L.; Liu, L. B.; Wang, S.; Zhu, D. B. Chem. Soc. Rev. 2010, 39, 2411. |
| [2] | Pankow, R. M.; Thompson, B. C. Polymer 2020, 207, 23. |
| [3] | Zhu, C. L.; Liu, L. B.; Yang, Q.; Lv, F. T.; Wang, S. Chem. Rev. 2012, 112, 4687. |
| [4] | Xu, W. D.; Lai, W. Y.; Fan, Q. L.; Huang, W. Sci. Sin. Chim. 2011, 41, 409. (in Chinese) |
| [4] | (徐巍栋, 赖文勇, 范曲立, 黄维, 中国科学:化学, 2011, 41, 409.) |
| [5] | Zhang, E. D.; Liu, L. B.; Lv, F. T.; Wang, S. Acta Polym. Sin. 2018, (2), 186. (in Chinese) |
| [5] | (张恩东, 刘礼兵, 吕凤婷, 王树, 高分子学报, 2018, (2), 186.) |
| [6] | Du, J. P.; Qin, P. J.; Xu, L. C.; Feng, S. S.; Xu, Y. X.; Huang, J. Chin. J. Org. Chem. 2020, 40, 194. (in Chinese) |
| [6] | (杜俊平, 秦鹏举, 绪连彩, 封珊珊, 徐云祥, 黄江, 有机化学, 2020, 40, 194.) |
| [7] | Liu, X. F.; Wang, Y. T.; Huang, Y. Q.; Feng, X. M.; Fan, Q. L.; Huang, W. Acta Chim. Sinica 2016, 74, 664. (in Chinese) |
| [7] | (刘兴奋, 王亚腾, 黄艳琴, 冯晓苗, 范曲立, 黄维, 化学学报, 2016, 74, 664.) |
| [8] | Zhou, X. Y.; Zhao, J. W.; Ma, G. M.; Jia, H. X. Chin. J. Chem. 2019, 47, 1006. |
| [9] | Al-Attar, H. A.; Monkman, A. P. Adv. Funct. Mater. 2012, 22, 3824. |
| [10] | Li, Z. L.; Acharya, R.; Wang, S. S.; Schanze, K. S. J. Mater. Chem. C 2018, 6, 3722. |
| [11] | Jagadesan, P.; Yu, Z. Q.; Barboza-Ramos, I.; Lara, H. H.; Vazquez-Munoz, R.; Lopez-Ribot, J. L.; Schanze, K. S. Chem. Mater. 2020, 32, 6186. |
| [12] | Liu, X. F.; Cai, X. H.; Huang, Y. Q.; Shi, L.; Fan, Q. L.; Huang, W. Acta Chim. Sinica 2014, 72, 440. (in Chinese) |
| [12] | (刘兴奋, 蔡小慧, 黄艳琴, 石琳, 范曲立, 黄维, 化学学报, 2014, 72, 440.) |
| [13] | Alizadeh, N.; Akbarinejad, A.; Hosseinkhani, S.; Rabbani, F. Anal. Chim. Acta 2019, 1084, 99. |
| [14] | Lv, S. Y.; Li, L.; Mu, Y. B.; Wan, X. B. Polym. Rev 2021, 61, 520. |
| [15] | Hu, Z. C.; Wang, Z. F.; Zhang, X.; Tang, H. R.; Liu, X. C.; Huang, F.; Cao, Y. iScience 2019, 13, 33. |
| [16] | Yin, C.; Tai, X. Y.; Li, X. Z.; Tan, J. H.; Lee, C. S.; Sun, P. F.; Fan, Q. L.; Huang, W. Chem. Eng. J. 2022, 428, 9. |
| [17] | Pei, T.; Peng, K.; Cai, X. Y.; Yuan, L. J.; Xia, J. B. Acta Phys.-Chim. Sin. 2017, 33, 2550 (In Chinese). |
| [17] | (裴童, 彭凯, 蔡心怡, 袁良杰, 夏江滨, 物理化学学报, 2017, 33, 2550.) |
| [18] | Cui, Q. L.; Xu, J. S.; Shen, G. Z.; Zhang, C.; Li, L. D.; Antonietti, M. ACS Appl. Mater. Inter. 2017, 9, 43966. |
| [19] | Pal, S.; Roy, D.; Mondal, M. K.; Chowdhury, P. J. Polym. Res. 2019, 26, 9. |
| [20] | Long, S.; Wan, Y.; Xia, A. D. Acta Chim. Sinica 2015, 73, 723. (in Chinese) |
| [20] | (龙飒然, 宛岩, 夏安东, 化学学报, 2015, 73, 723.) |
| [21] | Cui, Q. L.; He, F.; Wang, X. Y.; Xia, B. H.; Li, L. D. Abstracts of 2013 National Polymer Academic Paper Conference, Photoelectric Functional Polymer, Polymer Discipline Committee of Chinese Chemical Society, Chinese Chemical Society, 2013, p. 15. (in Chinese) |
| [21] | (崔倩玲, 贺芳, 王晓瑜, 夏碧华, 李立东, 2013年全国高分子学术论文报告会论文摘要集, 中国化学会, 2013, p. 15.) |
| [22] | Zhu, S. X.; Wang, X. Y.; Yang, Y.; Bai, H. T.; Cui, Q. L.; Sun, H.; Li, L. D.; Wang, S. Chem. Mater. 2018, 30, 3244. |
| [23] | Donabedian, P. L.; Creyer, M. N.; Monge, F. A.; Schanze, K. S.; Chi, E. Y.; Whitten, D. G. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 7278. |
| [24] | Tang, Y. L.; Zhou, Z. J.; Ogawa, K.; Lopez, G. P.; Schanze, K. S.; Whitten, D. G. J. Photochem. Photobiol. A-Chem. 2009, 207, 4. |
| [25] | Hill, E. H.; Sanchez, D.; Evans, D. G.; Whitten, D. G. Langmuir 2013, 29, 15732. |
| [26] | Hill, E. H.; Zhang, Y.; Evans, D. G.; Whitten, D. G. ACS Appl. Mater. Inter. 2015, 7, 5550. |
| [27] | Wurthner, F.; Kaiser, T. E.; Saha-Moller, C. R. Angew. Chem., Int. Ed. 2011, 50, 3376. |
| [28] | Du, C. S.; Gao, D.; Gao, M. S.; Yuan, H. B.; Liu, X. N.; Wang, B.; Xing, C. F. ACS Appl. Mater. Inter. 2021, 13, 27955. |
| [29] | Geoghegan, M.; Krausch, G. Prog. Polym. Sci. 2003, 28, 261. |
| [30] | Wei, H. T.; Jin, G.; Wang, L.; Hao, L.; Na, T. Y.; Wang, Y.; Tian, W. J.; Sun, H. Z.; Zhang, H. X.; Wang, H. Y.; Zhang, H.; Yang, B. Adv. Mater. 2014, 26, 3655. |
| [31] | Gu, Z.; Shen, Q. D.; Zhang, J.; Yang, C. Z.; Bao, Y. J. J. Appl. Polym. Sci. 2006, 100, 2930. |
| [32] | Knaapila, M.; Stewart, B.; Costa, T.; Rogers, S. E.; Pragana, J.; Fonseca, S. M.; Valente, A. J. M.; Ramos, M. L.; Murtinho, D.; Pereira, J. C.; Mallavia, R.; Burrows, H. D. Macromolecules 2016, 49, 9119. |
| [33] | Al-Attar, H. A.; Monkman, A. P. Phys. Rev. B 2012, 86, 235420. |
| [34] | Brustolin, F.; Goldoni, F.; Meijer, E. W.; Sommerdijk, N. A. J. M. Macromolecules 2002, 35, 1054. |
| [35] | Yuan, H. B.; Zang, Y.; Rowan, A. E.; Kouwer, P. H. J.; Xing, C. F. Angew. Chem., Int. Ed. 2020, 59, 2720. |
| [36] | Ho, H. A.; Boissinot, M.; Bergeron, M. G.; Corbeil, G.; Dore, K.; Boudreau, D.; Leclerc, M. Angew. Chem., Int. Ed. 2002, 41, 1548. |
| [37] | Park, S. Y.; Baik, H. J.; Oh, Y. T.; Oh, K. T.; Youn, Y. S.; Lee, E. S. Angew. Chem., Int. Ed. 2011, 50, 1644. |
/
| 〈 |
|
〉 |