

基于联萘酚骨架的新型圆偏振发光材料的合成及性能探究
Synthesis and Properties of Novel Circularly Polarized Luminescence Materials Based on Binaphthol Skeleton
Received date: 2022-03-19
Online published: 2022-04-25
Supported by
National Natural Science Foundation of China(21772012)
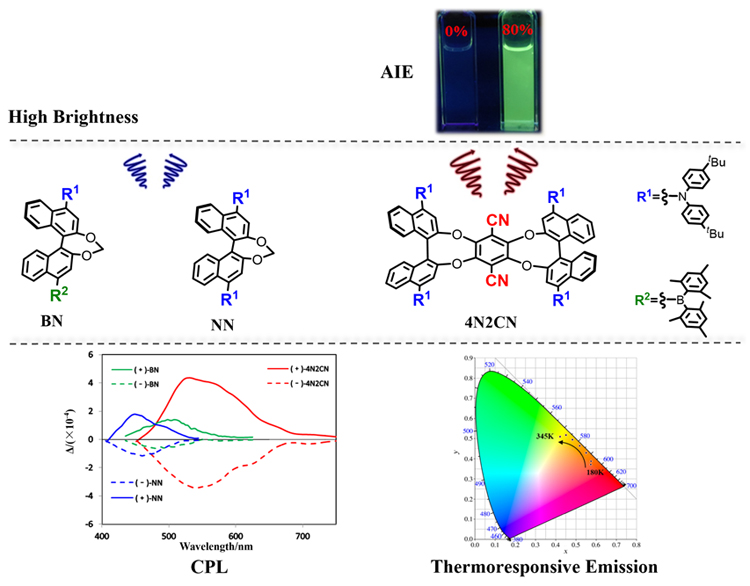
合成了基于联萘酚骨架并具有电子给体二芳基胺(NN)、电子给受体二芳基胺和二芳基硼(BN)、电子给受体二芳基胺和氰基(4N2CN)的系列手性发光分子, 通过引入不同的受体基团和扩展分子的手性骨架来比较几个分子的性质差异. 由于分子内电荷转移(ICT)特性, 化合物BN和4N2CN表现出显著的温度响应型荧光发射位移. 二芳基硼的引入使得BN在常见有机溶剂中的荧光量子效率相比NN明显提高并表现出更显著的溶剂化变色效应. 为了探索它们的手性光学性质, 三个消旋体化合物在光学提纯后进行圆二色光谱(CD)和圆偏振发光光谱(CPL)测试. 因为化合物4N2CN手性骨架的扩展, 其CPL信号和发光不对称因子(glum)优于NN. 这项工作可能有助于开发新型手性发光材料, 也为增强分子激发态的手性信号提供了一种思路.
刘斌 , 陈磅宽 . 基于联萘酚骨架的新型圆偏振发光材料的合成及性能探究[J]. 化学学报, 2022 , 80(7) : 929 -935 . DOI: 10.6023/A22030122
A series of chiral luminescent molecules based on binaphthol skeleton and having electron donor diarylamine (NN), electron donor/acceptor diarylamine and diarylboron (BN), electron donor/acceptor diarylamine and cyano (4N2CN) were synthesized. The properties of several molecules were compared by introducing different acceptor groups and extended molecular chiral skeletons. Compounds BN and 4N2CN exhibit significant thermochromic shift of the emission due to the intramolecular charge transfer (ICT) character. The emission wavelength was linearly enhanced with a high correlation coefficient of 0.991 for BN between 345 K and 150 K, 0.996 for 4N2CN between 345 K and 180 K. Furthermore, a fully reversible emission response was corroborated by an excellent fatigue resistance without emission degradation upon its exposure to 5 cycles of temperature change over a range of 195 K and 165 K for BN and 4N2CN. Optical resolution of the racemic mixtures have been performed for further investigation. The initial injection of these molecules into a chiral high performance liquid chromatography (HPLC) with a Daicel Chiralpak IA column resulted in two separated peaks with 1∶1 area. We try to further explore their chiral optical properties, including circular dichroism (CD) and circular polarized luminescence (CPL). The signal of CD indicates their chiral properties in the ground state. However, the difference of CPL signals of these compounds shows that the difference of group and skeleton will affect the chiral properties of molecules in the excited state. BN showed high brightness and ΦL of BN in solution can reach 0.99. In addition, 4N2CN exhibit aggregation-induced emission (AIE) properties in polar solvent and dynamic light scattering (DLS) was employed to monitor the process of aggregate formation. Based on this, we believe that the skeleton expansion and the introduction of electron-donor/acceptor groups make 4N2CN have unique photophysical properties. This work not only expands the family of chiral optical structures but also provides an idea to enhance the CPL signal and glum values.

| [1] | (a) Mandal, P. K.; Collie, G. W.; Kauffmann, B.; Huc, I. Angew. Chem., Int. Ed. 2014, 53, 14424. |
| [1] | (b) Xiao, W.; Ernst, K. H.; Palotas, K.; Zhang, Y.; Bruyer, E.; Peng, L.; Greber, T.; Hofer, W. A.; Scott, L. T.; Fasel, R. Nat. Chem. 2016, 8, 326. |
| [1] | (c) Gonźalez-Rubio, G.; Liz-Marzán, L. M. Nature 2018, 556, 313. |
| [2] | (a) Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. J. Am. Chem. Soc. 2014, 136, 5555. |
| [2] | (b) Cruz, C. M.; CastroFernández, S.; Mac?ôas, E.; Cuerva, J. M.; Campan?a, A. G. Angew. Chem., Int. Ed. 2018, 57, 14782. |
| [2] | (c) Han, X.-N.; Han, Y.; Chen, C.-F. J. Am. Chem. Soc. 2020, 142, 8262. |
| [2] | (d) Li, M.; Lin, W.-B.; Fang, L.; Chen, C.-F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese) |
| [2] | (李猛, 林伟彬, 房蕾, 陈传峰, 化学学报, 2017, 75, 1150.) |
| [2] | (e) Song, L.-F.; Zhou, Y.-Y.; Gao, T.; Yan, P.-F.; Li, H.-F. Acta Chim. Sinica 2021, 79, 1042. (in Chinese) |
| [2] | (宋龙飞, 周妍妍, 高婷, 闫鹏飞, 李洪峰, 化学学报, 2021, 79, 1042.) |
| [3] | (a) Carr, R.; Evans, N. H.; Parker, D. Chem. Soc. Rev. 2012, 41, 7673. |
| [3] | (b) Takaishi, K.; Yasui, M.; Ema, T. J. Am. Chem. Soc. 2018, 140, 5334. |
| [3] | (c) Ma, J.-L.; Peng, Q.; Zhao, C.-H. Chem. - Eur. J. 2019, 25, 15441. |
| [4] | (a) Grell, M.; Oda, M.; Whitehead, K. S.; Asimakis, A.; Neher, D.; Bradley, D. D. C. Adv. Mater. 2001, 13, 577. |
| [4] | (b) Li, M.; Li, S.-H.; Zhang, D.; Cai, M.; Duan, L.; Fung, M.-K.; Chen, C.-F. Angew. Chem., Int. Ed. 2018, 57, 2889. |
| [4] | (c) Zinna, F.; Voci, S.; Arrico, L.; Brun, E.; Homberg, A.; Bouffier, L.; Funaioli, T.; Lacour, J.; Sojic, N.; Di Bari, L. Angew. Chem., Int. Ed. 2019, 58, 6952. |
| [4] | (d) Liang, Z.-P.; Tang, R.; Qiu, Y.-C.; Wang, Y.; Lu, H.-B.; Wu, Z.-G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese) |
| [4] | (梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.) |
| [5] | (a) Zinna, F.; Giovanella, U.; Bari, L. D. Adv. Mater. 2015, 27, 1791. |
| [5] | (b) Brandt, J. R.; Salerno, F.; Fuchter, M. J. Nat. Rev. Chem. 2017, 1, 0045. |
| [5] | (c) Song, F.; Xu, Z.; Zhang, Q.; Zhao, Z.; Zhang, H.; Zhao, W.; Qiu, Z.; Qi, C.; Zhang, H.; Sung, H. H. Y.; Williams, I. D.; Lam, J. W. Y.; Zhao, Z.; Qin, A.; Ma, D.; Tang, B. Z. Adv. Funct. Mater. 2018, 28, 1800051. |
| [6] | (a) Tang, Y.; Cohen, A. E. Science 2011, 332, 333. |
| [6] | (b) Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Chem. - Eur. J. 2017, 23, 9249. |
| [6] | (c) He, C.; Yang, G.; Kuai, Y.; Shan, S.; Yang, L.; Hu, J.; Zhang, D.; Zhang, Q.; Zou, G. Nat. Commun. 2018, 9, 5117. |
| [7] | (a) Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. J. Am. Chem. Soc. 2019, 141, 11852. |
| [7] | (b) Jiang, Q.; Xu, X.; Yin, P.-A.; Ma, K.; Zhen, Y.; Duan, P.; Peng, Q.; Chen, W.-Q.; Ding, B. J. Am. Chem. Soc. 2019, 141, 9490. |
| [7] | (c) Zhang, C.; Yan, Z.-P.; Dong, X.-Y.; Han, Z.; Li, S.; Fu, T.; Zhu, Y.-Y.; Zheng, Y.-X.; Niu, Y.-Y.; Zang, S.-Q. Adv. Mater. 2020, 32, 2002914. |
| [8] | (a) Han, J.; Duan, P.; Li, X.; Liu, M. J. Am. Chem. Soc. 2017, 139, 9783. |
| [8] | (b) Liang, X.; Liu, T.-T.; Yan, Z.-P.; Zhou, Y.; Su, J.; Luo, X.-F.; Wu, Z.-G.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L. Angew. Chem., Int. Ed. 2019, 58, 17220. |
| [8] | (c) Zhao, W.-L.; Li, M.; Lu, H.-Y.; Chen, C.-F. Chem. Commun. 2019, 55, 13793. |
| [9] | (a) Sanchez-Carnerero, E. M.; Moreno, F.; Maroto, B. L.; Agarrabeitia, A. R.; Ortiz, M. J.; Vo, B. G.; Muller, G.; de la Moya, S. J. Am. Chem. Soc. 2014, 136, 3346. |
| [9] | (b) Kumar, J.; Tsumatori, H.; Yuasa, J.; Kawai, T.; Nakashima, T. Angew. Chem., Int. Ed. 2015, 54, 5943. |
| [9] | (c) Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Chem. - Eur. J. 2017, 23, 9249. |
| [10] | (a) Field, J. E.; Muller, G.; Riehl, J. P.; Venkataraman, D. J. Am. Chem. Soc. 2003, 125, 11808. |
| [10] | (b) Sawada, Y.; Furumi, S.; Takai, A.; Takeuchi, M.; Noguchi, K.; Tanaka, K. J. Am. Chem. Soc. 2012, 134, 4080. |
| [10] | (c) Katayama, T.; Nakatsuka, S.; Hirai, H.; Yasuda, N.; Kumar, J.; Kawai, T.; Hatakeyama, T. J. Am. Chem. Soc. 2016, 138, 5210. |
| [10] | (d) Otani, T.; Tsuyuki, A.; Iwachi, T.; Someya, S.; Tateno, K.; Kawai, H.; Saito, T.; Kanyiva, K. S.; Shibata, T. Angew. Chem., Int. Ed. 2017, 56, 3906. |
| [10] | (e) Qiu, Z.; Ju, C.-W.; Frédéric, L.; Hu, Y.; Schollmeyer, D.; Pieters, G.; Mu?llen, K.; Narita, A. J. Am. Chem. Soc. 2021, 143, 4661. |
| [11] | (a) Morisaki, Y.; Gon, M.; Sasamori, T.; Tokitoh, N.; Chujo, Y. J. Am. Chem. Soc. 2014, 136, 3350. |
| [11] | (b) Gon, M.; Morisaki, Y.; Chujo, Y. J. Mater. Chem. C 2015, 3, 521. |
| [11] | (c) Chen, J.-F.; Yin, X.; Wang, B.; Zhang, K.; Meng, G.; Zhang, S.; Shi, Y.; Wang, N.; Wang, S.; Chen, P. Angew. Chem., Int. Ed. 2020, 59, 11267. |
| [12] | (a) Lee, S.; Kaib, P. S. J.; List, B. J. Am. Chem. Soc. 2017, 139, 2156. |
| [12] | (b) Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. J. Am. Chem. Soc. 2019, 141, 11852. |
| [12] | (c) Takaishi, K.; Iwachido, K.; Takehana, R.; Uchiyama, M.; Ema, T. J. Am. Chem. Soc. 2019, 141, 6185. |
| [13] | Kimoto, T.; Tajima, N.; Fujiki, M.; Imai, Y. Chem. Asian J. 2012, 7, 2836. |
| [14] | (a) Tsumatori, H.; Nakashima, T.; Kawai, T. Org. Lett. 2010, 12, 2362. |
| [14] | (b) Zhang, S.; Wang, Y.; Meng, F.; Dai, C.; Cheng, Y.; Zhu, C. Chem. Commun. 2015, 51, 9014. |
| [14] | (c) Zhang, K.; Zhao, J. Y.; Zhang, N.; Chen, J. F.; Wang, N.; Yin, X. D.; Zheng, X. Y.; Chen, P. K. J. Mater. Chem. C 2022, 10, 1816. |
| [15] | (a) Entwistle, C. D.; Marder, T. B. Angew. Chem., Int. Ed. 2002, 41, 2927. |
| [15] | (b) Zhou, G.; Ho, C.-L.; Wong, W.-Y.; Wang, Q.; Ma, D.; Wang, L.; Lin, Z.; Marder, T. B.; Beeby, A. Adv. Funct. Mater. 2008, 18, 499. |
| [15] | (c) Sun, Z.-B.; Liu, J.-K.; Yuan, D.-F.; Zhao, Z.-H.; Zhu, X.-Z.; Liu, D.-H.; Peng, Q.; Zhao, C.-H. Angew. Chem., Int. Ed. 2019, 58, 4840. |
| [15] | (d) Chen, J.-F.; Yin, X.; Zhang, K.; Zhao, Z.; Zhang, S.; Zhang, N.; Wang, N.; Chen, P. J. Org. Chem. 2021, 86, 12654. |
| [15] | (e) Xia, Z.-Q.; Shao, A.-D.; Li, Q.; Zhu, S.-Q.; Zhu, W.-H. Acta Chim. Sinica 2016, 74, 351. (in Chinese) |
| [15] | (夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351.) |
| [15] | (f) Wang, T.; Hua, X.; Yu, Y.; Yuan, Y.; Feng, M.; Jiang, Z. Chin. J. Org. Chem. 2019, 39, 1436. (in Chinese) |
| [15] | (王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436.) |
| [15] | (g) Yu, J.; Xiao, Y.; Chen, J. Chin. J. Org. Chem. 2019, 39, 3460. (in Chinese) |
| [15] | (俞佳, 肖雅方, 陈嘉雄, 有机化学, 2019, 39, 3460.) |
| [16] | (a) Zhao, W.; Zhuang, X.; Wu, D.; Zhang, F.; Gehrig, D.; Laquai, F.; Feng, X. J. Mater. Chem. A 2013, 1, 13878. |
| [16] | (b) Sudhakar, P.; Mukherjee, S.; Thilagar, P. Organometallics 2013, 32, 3129. |
| [17] | (a) Liu, Z.-Q.; Shi, M.; Li, F.-Y.; Fang, Q.; Chen, Z.-H.; Yi, T.; Huang, C.-H. Org. Lett. 2005, 7, 5481. |
| [17] | (b) Stahl, R.; Lambert, C.; Kaiser, C.; Wortmann, R.; Jakober, R. Chem. - Eur. J. 2006, 12, 2358. |
| [18] | (a) Yuan, Z.; Entwistle, C. D.; Collings, J. C.; Albesa-Jové, D.; Batsanov, A. S.; Howard, J. A. K.; Taylor, N. J.; Kaiser, H. M.; Kaufmann, D. E.; Poon, S.-Y.; Wong, W.-Y.; Jardin, C.; Fathallah, S.; Boucekkine, A.; Halet, J.-F.; Marder, T. B. Chem. - Eur. J. 2006, 12, 2758. |
| [18] | (b) Cao, D.; Liu, Z.; Fang, Q.; Xu, G.; Xue, G.; Liu, G.; Yu, W. J. Organomet. Chem. 2004, 689, 2201. |
| [18] | (c) Liu, Z.; Fang, Q.; Wang, D.; Cao, D.; Xue, G.; Yu, W.; Lei, H. Chem. - Eur. J. 2003, 9, 5074. |
| [19] | (a) Shirota, Y.; Kinoshita, M.; Noda, T.; Okumoto, K.; Ohara, T. J. Am. Chem. Soc. 2000, 122, 11021. |
| [19] | (b) Doi, H.; Kinoshita, M.; Okumoto, K.; Shirota, Y. Chem. Mater. 2003, 15, 1080. |
| [19] | (c) Hudson, Z. M.; Sun, C.; Helander, M. G.; Amarne, H.; Lu, Z. H.; Wang, S. Adv. Funct. Mater. 2010, 20, 3426. |
| [20] | (a) Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y. Adv. Mater. 2011, 23, 1137. |
| [20] | (b) Zhang, D.; Song, X.; Gillett, A. J.; Drummond, B. H.; Jones, S. T. E.; Li, G.; He, H.; Cai, M.; Credgington, D.; Duan, L. Adv. Mater. 2020, 32, 1908355. |
| [21] | MacLean, M. W.; Wood, T. K.; Wu, G.; Lemieux, R. P.; Crudden, C. M. Chem. Mater. 2014, 26, 5852. |
| [22] | Zang, E.-S.; Hou, X.-F.; Zang, Z.; Zhang, Y.-Q.; Wang, J.-J.; Yang, H.; You, J.-M.; Ju, P. J. Mater. Chem. C 2019, 7, 8404. |
| [23] | (a) Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361. |
| [23] | (b) Zhang, Y.; Li, D.; Li, Y.; Yu, J. Chem. Sci. 2014, 5, 2710. |
| [23] | (c) Gu, Y.; Zhao, Z.; Su, H.; Zhang, P.; Liu, J.; Niu, G.; Li, S.; Wang, Z.; Kwok, R. T. K.; Ni, X.-L.; Sun, J.; Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Sci. 2018, 9, 6497. |
| [23] | (d) Zhao, Z.; Zhang, H.; Lam, J. W. Y.; Tang, B. Z. Angew. Chem., Int. Ed. 2020, 59, 9888. |
| [24] | Zhang, Z.; Edkins, R. M.; Nitsch, J.; Fucke, K.; Steffen, A.; Longobardi, L. E.; Stephan, D. W.; Lambert, C.; Marder, T. B. Chem. Sci. 2015, 6, 308. |
/
| 〈 |
|
〉 |