

金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究
收稿日期: 2022-03-17
网络出版日期: 2022-06-14
基金资助
国家自然科学基金(21832003); 国家自然科学基金(21972061); 国家重点研发计划(2021YFA1500900); 江苏省前沿引领技术基础研究专项(BK20212005)
Structural Regulation of Metal Organic Framework-derived Hollow Carbon Nanocages and Their Lithium-Sulfur Battery Performance
Received date: 2022-03-17
Online published: 2022-06-14
Supported by
National Natural Science Foundation of China(21832003); National Natural Science Foundation of China(21972061); National Key Research and Development Program of China(2021YFA1500900); Natural Science Foundation of Jiangsu Province, Major Project(BK20212005)
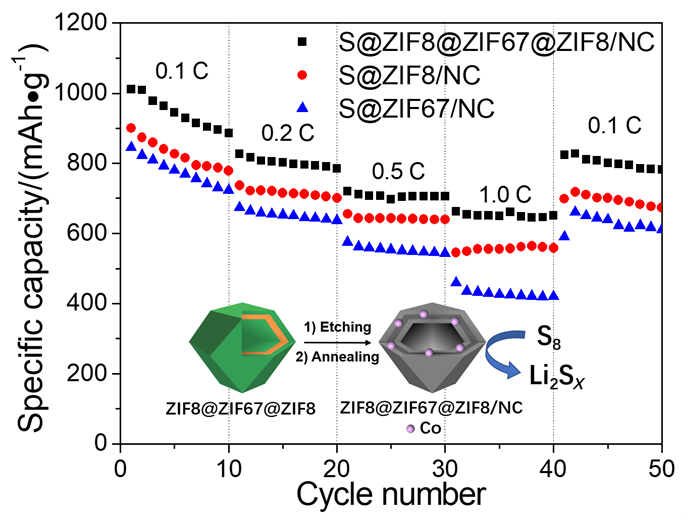
锂硫电池因其高理论比容量、低成本与环境友好等优点吸引了广泛的研究兴趣, 但实际应用仍受硫的利用率偏低、穿梭与极化效应严重等问题的制约. 本研究以金属有机框架材料为前体, 通过单宁酸蚀刻ZIF8、ZIF67和ZIF8@ZIF67@ZIF8颗粒产生空心内腔, 再经热解与碳化处理制得了粒径相近、笼壁组成与结构不同的三种空心碳纳米笼. 填充硫后用作锂硫电池的正极材料, ZIF8@ZIF67@ZIF8衍生的碳纳米笼展现出最优的性能, 在0.1 C时放电比容量达到1010 mAh•g-1, 在1 C时仍可保留664 mAh•g-1; 在0.5 C下循环300圈后仍可保留492 mAh•g-1, 显著优于ZIF8与ZIF67衍生的碳纳米笼对比样. 前者优异的性能源于其特殊的笼壁结构与组成: 前体中Co的存在可提高其导电性, Zn物种的蒸发带来大的比表面积和丰富的微孔/介孔, 有利于硫的填充以及Co物种与活性硫物种的接触及催化转化, 从而有效地抑制穿梭与极化效应, 提高正极中硫的利用率, 表现出更优的锂硫电池性能.
关键词: 锂硫电池; 正极材料; 金属有机框架衍生碳纳米笼; 电催化; 限域效应
何家伟 , 焦柳 , 程雪怡 , 陈光海 , 吴强 , 王喜章 , 杨立军 , 胡征 . 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022 , 80(7) : 896 -902 . DOI: 10.6023/A22030117
Lithium-sulfur battery has attracted extensive research interest because of its high theoretical specific capacity, low cost and environmental friendliness. However, its practical application is still limited by the low utilization of sulfur, serious shuttle and polarization effects. To solve these problems, confining sulfur in a highly conductive host, with the assistance of chemical adsorption of intermediate lithium polysulfides (LiPS) by polar sites and catalytic promotion of LiPS conversion by catalytic sites, can effectively boost the charge transfer and suppress the shuttle and polarization effects, hence leading to the high utilization of sulfur and the improved performances of lithium-sulfur batteries. Hollow carbon nanocages have become an ideal host for sulfur encapsulation because of the large inner cavity, high conductivity, and tunable surface and electronic structures. In this study, three kinds of hollow carbon nanocages with similar size but different composition and structure of shells were prepared by etching the ZIF8, ZIF67 and ZIF8@ZIF67@ZIF8 precursors with tannic acid solution, followed by the carbonization. Used as the cathodes for lithium-sulfur batteries after sulfur filling, the sample derived from ZIF8@ZIF67@ZIF8 shows the best electrochemical performance. Specifically, the specific capacity reaches 1010 mAh•g-1 at 0.1 C (1 C=1675 mA•g-1), and remains 664 mAh•g-1 at 1 C; after 300 cycles tests at 0.5 C, the capacity is retained at 492 mAh•g-1, significantly better than the control samples derived from ZIF8 and ZIF67. The excellent performance of the former is closely related to its unique structure and composition: (ⅰ) the Co species presented in the precursor can improve the conductivity of derived carbon nanocages; (ⅱ) the evaporation of Zn species brings about large specific surface area and rich micro/mesopores, which is conducive to the sulfur filling and the catalytic conversion of S species by the active Co species. Therefore, the shuttle and polarization effects are efficiently suppressed and the utilization of sulfur is improved accordingly, leading to the better performances of lithium-sulfur batteries. This study opens a new avenue to regulate the performance of lithium-sulfur batteries by constructing novel carbon nanocages hosts based on the metal organic framework (MOF) precursors.

| [1] | Li, X.; Sun, X. L. Adv. Mater. 2018, 28, 1801323. |
| [2] | Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Angew. Chem. Int. Ed. 2013, 52, 13186. |
| [3] | Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Small 2019, 15, 1804786. |
| [4] | Wang, X.; Li, Y. B.; Du, L. Y.; Gao, F. J.; Wu, Q.; Yang, L. J.; Chen, Q.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2018, 76, 627. (in Chinese) |
| [4] | (王啸, 李有彬, 杜玲玉, 高福杰, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2018, 76, 627.) |
| [5] | Fang, R. P.; Zhao, S. Y.; Sun, Z. H.; Wang, W.; Cheng, H. M.; Li, F. Adv. Mater. 2017, 29, 1606823. |
| [6] | Diao, Y.; Xie, K.; Hong, X. B.; Xiong, S. Z. Acta Chim. Sinica 2013, 71, 508. (in Chinese) |
| [6] | (刁岩, 谢凯, 洪晓斌, 熊仕昭, 化学学报, 2013, 71, 508.) |
| [7] | Cheng, X.; Shen, Z.; Jiao, L.; Yang, L.; Wang, X.; Wu, Q.; Hu, Z. EnergyChem 2021, 3, 100066. |
| [8] | Dai, L.; Chang, D. W.; Baek, J.-B.; Lu, W. Small 2012, 8, 1130. |
| [9] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Sci. China Chem. 2020, 63, 665. |
| [10] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Adv. Mater. 2020, 32, 1904177. |
| [11] | Hu, Z.; Wu, Q.; Chen, Y. Q. In Frontiers of Advanced Materials Research in China: Annual Report (2021), Chemical Industry Press, Beijing, 2021, Chapter 9. (in Chinese) |
| [11] | (胡征, 吴强, 陈轶群, 中国新材料研究前沿报告(2021), 化学工业出版社, 北京, 2021, 第九章) |
| [12] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Acc. Chem. Res. 2017, 50, 435. |
| [13] | Zhang, J.; Wang, K.; Guo, S.; Wang, S.; Liang, Z.; Chen, Z.; Fu, J.; Xu, Q. ACS Appl. Mater. Interfaces 2014, 6, 2192. |
| [14] | Lyu, Z.; Xu, D.; Yang, L.; Che, R.; Feng, R.; Zhao, J.; Li, Y.; Wu, Q.; Wang, X.; Hu, Z. Nano Energy 2015, 12, 657. |
| [15] | Wang, L. W.; Feng, R.; Xia, J. Z.; Chen, S.; Wu, Q.; Yang, L. J.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2014, 72, 1070. (in Chinese) |
| [15] | (王立伟, 冯瑞, 夏婧竹, 陈盛, 吴强, 杨立军, 王喜章, 胡征, 化学学报, 2014, 72, 1070.) |
| [16] | Han, J.; Xu, G.; Ding, B.; Pan, J.; Dou, H.; MacFarlane, D. R. J. Mater. Chem. A 2014, 2, 5352. |
| [17] | Fang, X.; Zang, J.; Wang, X.; Zheng, M.-S.; Zheng, N. J. Mater. Chem. A 2014, 2, 6191. |
| [18] | Ke, F. S.; Wu, Y. S.; Deng, H. J. Solid State Chem. 2015, 223, 109. |
| [19] | Jiang, H.; Liu, X. C.; Wu, Y.; Shu, Y.; Gong, X.; Ke, F. S.; Deng, H. Angew. Chem. Int. Ed. 2018, 57, 3916. |
| [20] | Wu, Y.; Jiang, H.; Ke, F. S.; Deng, H. Chem. Asian J. 2019, 14, 3577. |
| [21] | Yang, S. J.; Kim, T.; Im, J. H.; Kim, Y. S.; Lee, K.; Jung, H.; Park, C. R. Chem. Mater. 2012, 24, 464. |
| [22] | Song, X. K.; Guo, L. L.; Liao, X. M.; Liu, J.; Sun, J. H.; Li, X. P. Small 2017, 13, 1700238. |
| [23] | Guan, B. Y.; Yu, L.; Lou, X. W. Adv. Sci. 2017, 4, 1700247. |
| [24] | Xiao, J.; Zhao, C.; Hu, C.; Xi, J.; Wang, S. J. Power Sources 2017, 348, 183. |
| [25] | Liu, S.; Wang, Z.; Zhou, S.; Yu, F.; Yu, M.; Chiang, C.-Y.; Zhou, W.; Zhao, J.; Qiu, J. Adv. Mater. 2017, 29, 1700874. |
| [26] | Liu, C.; Huang, X.; Wang, J.; Song, H.; Yang, Y.; Liu, Y.; Li, J.; Wang, L.; Yu, C. Adv. Funct. Mater. 2018, 28, 1705253. |
| [27] | Wang, M. J.; Mao, Z. X.; Liu, L.; Peng, L.; Yang, N.; Deng, J.; Ding, W.; Li, J.; Wei, Z. Small 2018, 14, 1804183. |
| [28] | Zhang, W.; Jiang, X.; Zhao, Y.; Carne-Sanchez, A.; Malgras, V.; Kim, J.; Kim, J. H.; Wang, S.; Liu, J.; Jiang, J. S.; Yamauchi, Y.; Hu, M. Chem. Sci. 2017, 8, 3538. |
| [29] | Abdul Nasir Khan, M.; Kwame Klu, P.; Wang, C.; Zhang, W.; Luo, R.; Zhang, M.; Qi, J.; Sun, X.; Wang, L.; Li, J. Chem. Eng. J. 2019, 363, 234. |
| [30] | Wang, Q.; Zhang, Z.; Shi, S.; Wu, F.; Zhang, Z.; Li, G.; Suo, Y. J. Electroanal. Chem. 2021, 894, 115397. |
| [31] | Wu, H.; Qian, X.; Zhu, H.; Ma, S.; Zhu, G.; Long, Y. RSC Adv. 2016, 6, 6915. |
| [32] | Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; Shao, Y.; Wu, G. J. Am. Chem. Soc. 2017, 139, 14143. |
| [33] | Chen, H.; Shen, K.; Tan, Y.; Li, Y. ACS Nano 2019, 13, 7800. |
| [34] | Tang, J.; Salunkhe, R. R.; Liu, J.; Torad, N. L.; Imura, M.; Furukawa, S.; Yamauchi, Y. J. Am. Chem. Soc. 2015, 137, 1572. |
/
| 〈 |
|
〉 |