

基于分子间卤键的超分子平面大环自组装
收稿日期: 2022-08-30
网络出版日期: 2022-10-08
基金资助
河南省高等学校重点科研项目计划(21A150045); 商丘师范学院博士启动经费(700200); 商丘师范学院博士启动经费(700205)
Self-assembly of Supramolecular Planar Macrocycle Driven by Intermolecular Halogen Bonding
Received date: 2022-08-30
Online published: 2022-10-08
Supported by
Key Scientific Research Projects of Colleges and Universities of Henan Province(21A150045); Start-up Foundation for Scientific Research of Newly Recruited PhD of Shangqiu Normal University(700200); Start-up Foundation for Scientific Research of Newly Recruited PhD of Shangqiu Normal University(700205)
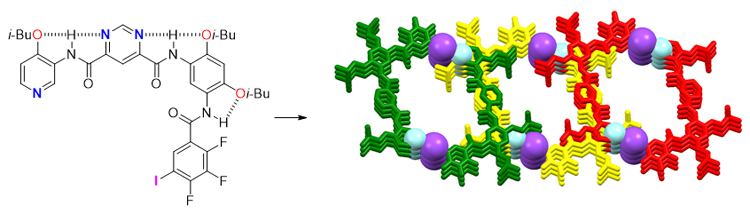
本工作报道了含卤键供体和受体片段的三种芳酰胺分子(化合物1~3)的设计和合成, 并对固相中卤键的不同作用模式进行了探索和分析. 化合物1的晶体数据显示, 由于没有分子内氢键, 组成分子的三个芳环相互扭转一定角度, 并且在分子间交替排列的N···I和O···I卤键的控制下, 组装成了一条线型的超分子组装体. 由于酰胺羰基和两个紧邻的氟原子之间的排斥作用, 化合物2未能形成分子内三中心氢键. 在此基础上, 将三氟碘代苯作为卤键供体片段引入到化合物3中, 并且在折叠体骨架中嵌入了嘧啶单元. 化合物3的晶体数据显示, 基于多组有效的分子内三中心氢键和分子间较强的卤键作用, 双分子间形成了[1+1]的超分子大环. 另外, 由于嘧啶环的引入, 使得该超分子大环接近共平面.
刘传志 , 李芬 , 王静静 , 赵晓璐 , 张婷美 , 黄鑫 , 邬梦丽 , 户志远 , 刘新明 , 黎占亭 . 基于分子间卤键的超分子平面大环自组装[J]. 化学学报, 2022 , 80(10) : 1365 -1368 . DOI: 10.6023/A22080368
The design and synthesis of three kinds of arylamide molecules (compounds 1~3) containing halogen bonding donor and acceptor fragments, and the exploration and analyzation of different action modes of halogen bonding in solid phase were reported. Compounds 1 and 2 contain two tetrafluoroiodobenzene fragments, and compound 1 also contains a halogen receptor fragment—pyridine group. Isobutyl groups are introduced into the molecule to increase its solubility and crystallinity. And a pyrimidine fragment was introduced into compound 3, which has more aromatic rings. The two N atoms of the pyrimidine fragment can theoretically form intramolecular hydrogen bonds with the adjacent amide hydrogen atoms (—C(=O)NH), so that the whole molecule has the properties of hydrogen-bonded arylamide foldamer. Moreover, trifluorobenzene fragments were selected in compound 3 to eliminate the repulsion between excess fluorine atoms and carbonyls. The crystal structures reveal that the three aromatic rings in compound 1 are twisted with each other for there is no intramolecular hydrogen bond, and a supramolecular DNA-like double helix was assembled controlled by intermolecular N···I and O···I halogen bonds arranged alternately. Compound 2 failed to form an intramolecular three-center hydrogen bonding due to the repulsion between the amide carbonyl groups and the two fluorine atoms in close proximity. As expected, in the solid phase of compound 3, an effective three-center hydrogen bond is formed between the terminal trifluoroiodobenzene and the benzene ring attached to it. Moreover, the two N—H bonds connected to the pyrimidine ring also form two effective three-center hydrogen bonds. The difference is that the participants of these two groups of three-center hydrogen bonds include two N atoms in the pyrimidine ring. The four aromatic rings in compound 3 are nearly coplanar driven by these intramolecular three-center hydrogen bonds. Two sets of strong intermolecular (pyridine ring) N···I halogen bonds control the formation of [1+1] bimolecular supramolecular macrocycles with inner diameters of 1.36 nm and 1.07 nm in length and width. Moreover, the supramolecular macrocycle is near-planar due to the introduction of pyrimidine ring.

| [1] | Politzer, P.; Lane, P.; Concha, M. C.; Ma, Y.; Murray, J. S. J. Mol. Model. 2007, 13, 305. |
| [2] | Rissanen, K. CrystEngComm 2008, 10, 1107. |
| [3] | Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. Acc. Chem. Res. 2013, 46, 2686. |
| [4] | Mukherjee, A.; Tothadi, S.; Desiraju, G. R. Acc. Chem. Res. 2014, 47, 2514. |
| [5] | Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478. |
| [6] | Fu, Y.; Xiang, Z.; Zhou, J.; Wu, X.; Li, Y.; Jiao, Y. Acta Chim. Sinica 2012, 70, 1847 ( in chinese). |
| [6] | (付昱, 向子龙, 周军, 吴欣蔚, 李妍, 焦永华, 化学学报, 2012, 70, 1847.) |
| [7] | Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Zhao, X.; Li, Z.-T. Chin. J. Org. Chem. 2019, 39, 28. (in Chinese) |
| [7] | (刘传志, 王辉, 张丹维, 赵新, 黎占亭, 有机化学, 2019, 39, 28.) |
| [8] | Ding, X.-H.; Chang, Y.-Z.; Ou, C.-Q.; Lin, J.-Y.; Xie, L.-H.; Huang, W. Natl. Sci. Rev. 2020, 7, 1906. |
| [9] | Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 7118. |
| [10] | Kianimehra, A.; Akhbaria, K.; Whiteb, J.; Phuruangratc, A. Inorg. Chem. Commun. 2020, 115, 107864. |
| [11] | Bulfield, D.; Huber, S. M. Chem. Eur. J. 2016, 22, 14434. |
| [12] | Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622. |
| [13] | Zhang, H.-M.; Toya, P. H. Adv. Synth. Catal. 2021, 363, 215. |
| [14] | Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem. Int. Ed. 2021, 60, 5069. |
| [15] | Ma, X.-K.; Zhang, W.; Liu, Z.; Zhang, H.; Zhang, B.; Liu, Y. Adv. Mater. 2021, 2007476. |
| [16] | Cao, J.; Yan, X.; He, W.; Li, X.; Li, Z.; Mo, Y.; Liu, M.; Jiang, Y.-B. J. Am. Chem. Soc. 2017, 139, 6605. |
| [17] | Zheng, J.; Suwardi, A.; Wong, C. J. E.; Loh, X. J.; Li, Z. B. Nanoscale Adv. 2021, 3, 6342. |
| [18] | Gong, G.-F.; Lv, S.-H.; Han, J.-X.; Xie, F.; Li, Q.; Xia, N.; Zeng, W.; Chen, Y.; Wang, L.; Wang, J.-K.; Chen, S.-G. Angew. Chem. Int. Ed. 2021, 60, 14831. |
| [19] | Borchers, T.-H.; Topić, F.; Christopherson, J. C.; Bushuyev, O.-S.; Vainauskas, J.; Titi, H. M.; Friščić, T.; Barrett, C. J. Nature Chem. 2022, 14, 574. |
| [20] | Montaña, Á. M. ChemistrySelect 2017, 2, 9094. |
| [21] | Sumii, Y.; Sasaki, K.; Tsuzuki, S.; Shibata, N. Molecules 2019, 24, 3610. |
| [22] | Zhu, Z.; Wang, G.; Xu, Z.; Chen, Z.; Wang, J.; Shi, J.; Zhu, W. Phys. Chem. Chem. Phys. 2019, 21, 15106. |
| [23] | Zhao, H.; Sheng, S.; Hong, Y.; Zeng, H. J. Am. Chem. Soc. 2014, 136, 14270. |
| [24] | Jentzsch, A. V.; Matile, S. Top. Curr. Chem. 2014, 358, 205. |
| [25] | Huo, Y. P.; Zeng, H. Q. Acc. Chem. Res. 2016, 49, 922. |
| [26] | Shen, J.; Fan, J. R.; Ye, R. J.; Li, N.; Mu, Y. G.; Zeng, H. Q. Angew. Chem. Int. Ed. 2020, 59, 13328. |
| [27] | Zheng, S.-P.; Huang, L.-B.; Sun, Z.-H.; Barboiu, M. Angew. Chem. Int. Ed. 2021, 60, 566. |
| [28] | Wang, C.-X.; Wang, S.-K.; Yang, H.-T.; Xiang, Y.-X.; Wang, X.-B.; Bao, C.-Y.; Zhu, L.-Y.; Tian, H.; Qu, D.-H. Angew. Chem. Int. Ed. 2021, 60, 14836. |
| [29] | Chen, S.-J.; Wang, Y.-C.; Nie, T.; Bao, C.-Y.; Wang, C.-X.; Xu, T.-Y.; Lin, Q.-N.; Qu, D.-H.; Gong, X.-Q.; Yang, Y.; Zhu, L.-Y.; Tian, H. J. Am. Chem. Soc. 2018, 140, 17992. |
| [30] | Pancholi, J.; Beer, P. D. Coord. Chem. Rev. 2020, 416, 213281. |
| [31] | Dong, S. Y.; Zheng, B.; Wang, F.; Huang, F. H. Acc. Chem. Res. 2014, 47, 1982. |
| [32] | Jungbauer, S. H.; Bulfield, D.; Kniep, F.; Lehmann, C. W.; Herdtweck, E.; Huber, S. M. J. Am. Chem. Soc. 2014, 136, 16740. |
| [33] | Gong, B. Chem.-Eur. J. 2001, 7, 4336. |
| [34] | Huc, I. Eur. J. Org. Chem. 2004, 17. |
| [35] | Li, Z.-T.; Hou, J.-L.; Li, C. Acc. Chem. Res. 2008, 41, 1343. |
| [36] | Roy, A.; Prabhakaran, P.; Baruah, P. K.; Sanjayan, G. J. Chem. Commun. 2011, 47, 11593. |
| [37] | Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Chem. Rev. 2012, 112, 5271. |
| [38] | Liu, C.-Z.; Yan, M.; Wang, H.; Zhang, D.-W.; Li, Z.-T. ACS Omega 2018, 3, 5165. |
| [39] | Sun, G.-J.; Nie, C.-B.; Zhao, X.; Li, Z.-T. Chin. J. Org. Chem. 2017, 37, 1757. (in Chinese) |
| [39] | (孙广军, 聂承斌, 赵新, 黎占亭, 有机化学, 2017, 37, 1757.) |
| [40] | Yang, L.; Zhao, W.; Che, Y.-K.; Wang, Y.; Jiang, H. Chin. Chem. Lett. 2017, 28, 1659. |
| [41] | Zhang, D.-W.; Wang, H.; Li, Z.-T. Macromol. Rapid Commun. 2017, 38, 1700179. |
| [42] | Liu, C.-Z.; Koppireddi, S.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Angew. Chem. Int. Ed. 2019, 58, 226. |
| [43] | Liu, C.-Z.; Koppireddi, S.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2019, 30, 953. |
| [44] | Koppireddi, S.; Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Li, Z.-T. CrystEngComm 2019, 21, 2626. |
| [45] | Xu, Y.-Y.; Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Org. Chem. 2021, 41, 2848. (in Chinese) |
| [45] | (许艳艳, 刘传志, 王辉, 张丹维, 黎占亭, 有机化学, 2021, 41, 2848.) |
| [46] | Yu, S.; Kalenius, E.; Frontera, A.; Rissanena, K. Chem. Commun. 2021, 57, 12464. |
| [47] | Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Angew. Chem. Int. Ed. 2008, 47, 6114. |
| [48] | Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 7118. |
| [49] | Wang, H.; Wang, W.; Jin, W.-J. Chem. Rev. 2016, 116, 5072. |
| [50] | Tepper, R.; Schubert, U. S. Angew. Chem. Int. Ed. 2018, 57, 6004. |
| [51] | Jiang, H.; Léger, J. M.; Huc, I. J. Am. Chem. Soc. 2003, 125, 3448. |
/
| 〈 |
|
〉 |