

调控供电子策略简易制备近红外二区有机小分子光学诊疗试剂
收稿日期: 2022-06-21
网络出版日期: 2022-10-09
基金资助
国家自然科学基金(22175098); 国家自然科学基金(21975131); 江苏省博士后科研资助计划(2021K114B); 江苏省高等学校自然科学研究面上项目(21KJB430031); 南京邮电大学校级科研基金(NY220198)
Simple Preparation of Near-infrared-II Organic Small Molecule-based Phototheranostics by Manipulation of the Electron-donating Unit
Received date: 2022-06-21
Online published: 2022-10-09
Supported by
National Natural Science Foundation of China(22175098); National Natural Science Foundation of China(21975131); Postdoctoral Science Foundation of Jiangsu Province(2021K114B); Natural Science Foundation of the Jiangsu Higher Education Institutions(21KJB430031); Scientific Fund of Nanjing University of Posts and Telecommunications(NY220198)
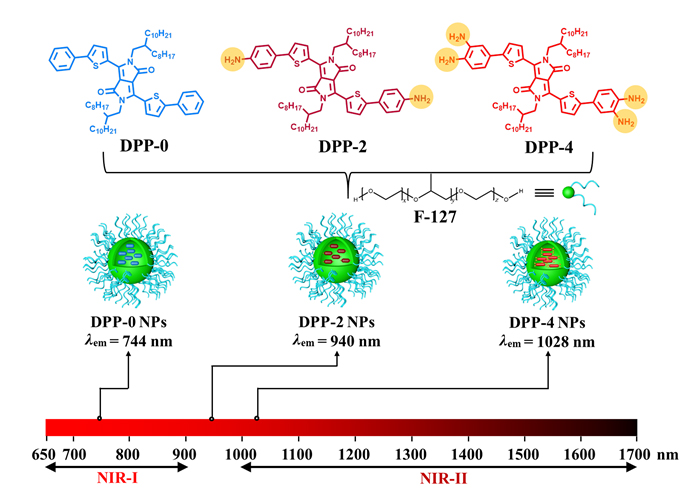
有机小分子凭借着明确的化学结构、出色的生物相容性、优异的可重复性等诸多优势被广泛应用于光学诊疗领域. 然而, 目前报道的有机小分子存在合成步骤复杂、成像波长位于近红外一区(NIR-I)、光热转换效率及单线态氧产率低等缺陷, 严重限制了其诊疗效果. 基于此, 本工作以吡咯并吡咯二酮作为缺电子单元、分别以苯、苯胺、邻苯二胺作为供电子单元, 通过一步偶联反应简易制备得到三种有机小分子DPP-0、DPP-2、DPP-4, 进一步利用纳米沉淀法制备得到对应的水溶性纳米粒子DPP-0 NPs、DPP-2 NPs和DPP-4 NPs. 研究发现, 随着氨基数量的增加, 纳米粒子吸收/发射均发生了红移, 其中DPP-4 NPs具有良好的NIR-I吸收能力且其最大荧光发射达到了近红外二区(NIR-II)区域, 表明可以通过改变供电子单元策略实现光学性能的调控. 在单一激光照射下, DPP-4 NPs可以同时产生NIR-II荧光信号、过高热及单线态氧, 其光热转换效率和单线态氧产率分别高达40.2%及34.3%, 可成功应用于肿瘤深层次NIR-II荧光成像诊断及高效光热/光动力联合治疗.
王其 , 夏辉 , 熊炎威 , 张新敏 , 蔡杰 , 陈冲 , 高逸聪 , 陆峰 , 范曲立 . 调控供电子策略简易制备近红外二区有机小分子光学诊疗试剂[J]. 化学学报, 2022 , 80(11) : 1485 -1493 . DOI: 10.6023/A22060267
Organic small molecules hold great promise in the field of phototheranostics by virtue of their clear chemical structure, outstanding biocompatibility, and distinguished repeatability. Whereas the synthetic process of them suffers from multi-step and complex reactions. Meanwhile, most of small molecules employed near-infrared-I (NIR-I) fluorescence imaging, which could not effectively provide comprehensive tumor information with high resolution. In addition, the reported small molecules showed unsatisfactory singlet oxygen yield and photothermal conversion efficiency, limiting their extensive application in cancer theranostics. Herein, by conjugating diketopyrrolopyrrole (an electron-withdrawing unit) with benzene, aniline, or 1,2-diaminobenzene (electron-donating units), respectively, three novel small molecules DPP-0, DPP-2 and DPP-4 were designed and synthesized by one-step reaction. DPP-0 NPs, DPP-2 NPs and DPP-4 NPs as phototheranostics were further obtained through nanoprecipitation method by self-assembling with Pluronic F127. It was found that with the increase of amino groups, the absorption/emission peaks of NPs were red-shifted. Especially, DPP-4 NPs exhibited strong absorption in the NIR-I region, and the maximum emission peak of DPP-4 NPs was located in the near-infrared-II (NIR-II) region, suggesting that the optical properties of small molecules can be regulated by changing the electron-donating unit. After irradiation of DPP-4 NPs with a single laser, excellent NIR-II fluorescence signal can be obtained for efficient imaging of tumor with high resolution. Moreover, abundant reactive oxygen species (singlet oxygen yield 34.3%) and hyperthermia (photothermal conversion efficiency 40.2%) can also be produced by irradiation of DPP-4 NPs, realizing the combined photodynamic/photothermal therapy guided by NIR-II fluorescence imaging.

| [1] | (a) Li, J.; Pu, K. Acc. Chem. Res. 2020, 53, 752. |
| [1] | (b) Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Adv. Mater. 2022, 34, 2107434. |
| [2] | (a) Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Angew. Chem. Int. Ed. 2021, 60, 16763. |
| [2] | (b) Duan, X.; Zhang, Q.; Jiang, Y.; Wu, X.; Yue, X.; Geng, Y.; Shen, J.; Ding, D. Adv. Mater. 2022, 34, 2200179. |
| [2] | (c) Xue, L.; Shen, Q.; Zhang, T.; Fan, Y. B.; Xu, X. G.; Shao, J. J.; Yang, D. L.; Zhao, W. L.; Dong, X. C.; Mou, X. Z. Mater. Chem. Front. 2021, 5, 6061. |
| [3] | (a) Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Chem. Soc. Rev. 2021, 50, 4185. |
| [3] | (b) Xu, Y.; Tuo, W.; Yang, L.; Sun, Y.; Li, C.; Chen, X.; Yang, W.; Yang, G.; Stang, P. J.; Sun, Y. Angew. Chem. Int. Ed. 2022, 61, 202110048. |
| [3] | (c) Yang, Z.; Zhu, Y.; Dong, Z.; Hao, Y.; Wang, C.; Li, Q.; Wu, Y.; Feng, L.; Liu, Z. Biomaterials 2022, 281, 121332. |
| [3] | (d) Li, Y.; Wang, Z.; Tang, Z. Acta Chim. Sinica 2022, 80, 291. (in Chinese) |
| [3] | (李嫣然, 王子贵, 汤朝晖, 化学学报, 2022, 80, 291.) |
| [4] | (a) Yu, Z.; Chan, W. K.; Zhang, Y.; Tan, T. T. Y. Biomaterials 2021, 269, 120459. |
| [4] | (b) Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; Shi, B.; Tang, B. Z. Adv. Mater. 2022, 34, 2106082. |
| [4] | (c) Men, X.; Yuan, Z. ACS Appl. Polym. Mater. 2020, 2, 4319. |
| [4] | (d) Xu, C.; Pu, K. Chem. Soc. Rev. 2021, 50, 1111. |
| [4] | (e) Zhang, S.; Feng, S.; Ma, L.; Yang, Y.; Liu, C.; Song, N.; Yang, Y. Acta Chim. Sinica 2022, 80, 265. (in Chinese) |
| [4] | (张审, 冯闪, 马陇豫, 杨莹莹, 刘超群, 宋宁宁, 杨彦伟, 化学学报, 2022, 80, 265.) |
| [5] | (a) Wang, Q.; Niu, X.; Yang, L.; Liu, J.; Wang, J.; Xu, X.; Tang, W.; Huang, W.; Fan, Q. Mater. Chem. Front. 2021, 5, 5689. |
| [5] | (b) Dai, H.; Wang, X.; Shao, J.; Wang, W.; Mou, X.; Dong, X. Small 2021, 17, 2102646. |
| [5] | (c) Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N. T.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Adv. Mater. 2020, 32, 2004481. |
| [6] | (a) Sun, C.; Sun, X.; Pei, P.; He, H.; Ming, J.; Liu, X.; Liu, M.; Zhang, Y.; Xia, Y.; Zhao, D.; Li, X.; Xie, Y.; Zhang, F. Adv. Funct. Mater. 2021, 31, 2100656. |
| [6] | (b) Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. J. Am. Chem. Soc. 2020, 142, 14789. |
| [6] | (c) Yuan, Y.; Feng, Z.; Li, S.; Huang, Z.; Wan, Y.; Cao, C.; Lin, S.; Wu, L.; Zhou, J.; Liao, L. S.; Qian, J.; Lee, C. S. Adv. Mater. 2022, 34, 2201263. |
| [6] | (d) Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; Qiao, X.; Gao, J.; Wang, X.; Deng, Z.; Meng, X.; Xiao, Y.; Kim, J. S.; Hong, X. Chem. Rev. 2022, 122, 209. |
| [6] | (e) Lu, F.; Sang, R.; Tang, Y.; Xia, H.; Liu, J.; Huang, W.; Fan, Q.; Wang, Q. Acta Biomater. 2022, 151, 528. |
| [7] | (a) Yang, H.; Huang, H.; Ma, X.; Zhang, Y.; Yang, X.; Yu, M.; Sun, Z.; Li, C.; Wu, F.; Wang, Q. Adv. Mater. 2021, 33, 2103953. |
| [7] | (b) Wu, D.; Liu, S.; Zhou, J.; Chen, R.; Wang, Y.; Feng, Z.; Lin, H.; Qian, J.; Tang, B. Z.; Cai, X. ACS Nano 2021, 15, 5011. |
| [7] | (c) Yang, Q.; Ma, H.; Liang, Y.; Dai, H. Acc. Mater. Res. 2021, 2, 170. |
| [7] | (d) He, S.; Song, J.; Qu, J.; Cheng, Z. Chem. Soc. Rev. 2018, 47, 4258. |
| [7] | (e) Xiong, L.; Fan, Y.; Zhang, F. Acta Chim. Sinica 2019, 77, 1239. (in Chinese) |
| [7] | (熊麟, 凡勇, 张凡, 化学学报, 2019, 77, 1239.) |
| [8] | Peng, F.; Setyawati, M. I.; Tee, J. K.; Ding, X.; Wang, J.; Nga, M. E.; Ho, H. K.; Leong, D. T. Nat. Nanotechnol. 2019, 14, 279. |
| [9] | (a) Feng, G.; Zhang, G.; Ding, D. Chem. Soc. Rev. 2020, 49, 8179. |
| [9] | (b) Kenry |
| [9] | (c) Mu, J.; Xiao, M.; Shi, Y.; Geng, X.; Li, H.; Yin, Y.; Chen, X. Angew. Chem. Int. Ed. 2022, 61, 202114722. |
| [9] | (d) Sang, R.; Xu, X.; Wang, Q.; Fan, Q.; Huang, W. Acta Chim. Sinica 2020, 78, 901. (in Chinese) |
| [9] | (桑若愚, 许兴鹏, 王其, 范曲立, 黄维, 化学学报, 2020, 78, 901.) |
| [10] | Yin, C.; Tai, X.; Li, X.; Tan, J.; Lee, C.-S.; Sun, P.; Fan, Q.; Huang, W. Chem. Eng. J. 2022, 428, 132098. |
| [11] | Chen, J.; Wen, K.; Chen, H.; Jiang, S.; Wu, X.; Lv, L.; Peng, A.; Zhang, S.; Huang, H. Small 2020, 16, 2000909. |
| [12] | (a) Wang, Q.; Xu, J.; Geng, R.; Cai, J.; Li, J.; Xie, C.; Tang, W.; Shen, Q.; Huang, W.; Fan, Q. Biomaterials 2020, 231, 119671. |
| [12] | (b) Xu, Y.; Zhang, Y.; Li, J.; An, J.; Li, C.; Bai, S.; Sharma, A.; Deng, G.; Kim, J. S.; Sun, Y. Biomaterials 2020, 259, 120315. |
| [12] | (c) Li, Y.; Fan, X.; Li, Y.; Liu, S.; Chuah, C.; Tang, Y.; Kwok, R. T. K.; Lam, J. W. Y.; Lu, X.; Qian, J.; Tang, B. Z. ACS Nano 2022, 16, 3323. |
| [12] | (d) Su, Y.; Yu, B.; Wang, S.; Cong, H.; Shen, Y. Biomaterials 2021, 271, 120717. |
/
| 〈 |
|
〉 |