

二氟环丙烯参与的有机反应研究进展★
收稿日期: 2023-03-21
网络出版日期: 2023-04-20
基金资助
受国家自然科学基金(22171206); 浙江省自然科学基金(Z23B020002)
Recent Advances in the Transformation of Difluorocyclopropenes★
Received date: 2023-03-21
Online published: 2023-04-20
Supported by
National Natural Science Foundation of China(22171206); Natural Science Foundation of Zhejiang Province(Z23B020002)
黄家翩 , 刘飞 , 吴劼 . 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023 , 81(5) : 520 -532 . DOI: 10.6023/A23030088
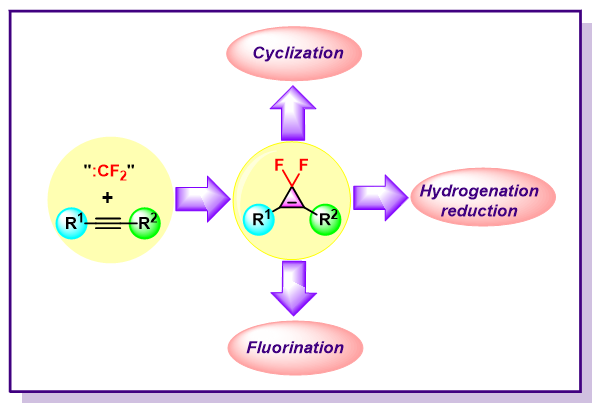
In recent years, organic reactions involving difluorocyclopropenes have attracted the attention of organic chemists and have made great progress. The reactions mainly include: (1) cyclization: a) transition-metal catalyzed C—H bond activation cyclization with directing groups; b) cyclization reactions with non-directing groups; (2) hydrogenation reduction; (3) fluorination as “F” source reagents. In this paper, the synthesis methods and applications of difluorocyclopropenes in past 10 years are summarized. The conversion reactions of difluorocyclopropenes are emphasized. Additionally, difluorocyclopropenes have aroused considerable interests both from a structural standpoint and their participation in various ring-opening reactions. Given the increasing application of cyclopropyl skeleton in the development of drugs and unarguable importance of fluorinated compounds in medicinal chemistry and agrochemistry, it is no doubt that difluorocyclopropenes are encountered into bioactive molecular and at present lie among “emerging fluorinated motifs”. Although the synthesis and application of structurally diverse difluorocyclopropenes have been witnessed in the past decade, the most widely used methods for the preparation of these compounds include difluoromethylenation of alkynes and difluoromethylation of heteroatom nucleophiles (such as NaF, NaI, nBuN4X, etc.) with a difluorocarbene reagent, which can be generated from various precursors (such as TMSCF3, TMSCF2X, Ph3P+CF2CO2-, TFDA, etc.). For the transition metal-catalyzed cyclization of difluorocyclopropenes, some common metal salts (such as rhodium, ruthenium, copper, palladium, silver) are used as catalysts. Moreover, the metallic hydrogen (M—H) reduction strategy is a simple and efficient method for the hydrogenation reduction of difluorocyclopropenes leading to difluorocyclopropanes, and the asymmetric hydrogenation reduction of difluorocyclopropenes can be achieved in the presence of chiral ligands. In fluorination reactions, difluorocyclopropenes have some advantages that cannot be achieved by traditional fluorination reagents for the direct fluorination and functionalization of hydroxyl groups (such as fluorination of polyhydroxyl alcohols). Of course, the biggest disadvantage of difluorocyclopropene as a fluorine source lies in its poor atomic economy, which has been criticized. Despite the remarkable achievements in the reactions of difluorocyclopropenes, there are still many issues that need to be addressed. For instance, difluorocyclopropenes are rarely applied in traditional radical reactions, photocatalysis, electrocatalysis and flow chemistry. Hopefully, difluorocyclopropenes can gradually appear in photo- and electro-catalyzed radical chemistry, and the related asymmetric reactions will also get more attention and development in the near future.
| [1] | Itoh, T. In Fluorine in Medicinal Chemistry and Chemical Biology, Ed.: Ojima, I., Wiley-Blackwell, Oxford, 2009, pp. 313-334. |
| [2] | (a) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013. |
| [2] | (b) Begue, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, NJ, 2008. |
| [2] | (c) Fluorine in Medicinal Chemistry and Chemical Biology, Ed.: Ojima, I., Wiley,Chichester, 2009. |
| [2] | (d) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. |
| [2] | (e) Song, S.; Xu, S. Chin. J. Org. Chem. 2023, 43, 411. (in Chinese) |
| [2] | 宋树勇, 徐森苗, 有机化学, 2023, 43, 411). |
| [2] | (f) Sun, Q.; Sun, Z.; Yu, Z.; Wang, G. Chin. J. Org. Chem. 2022, 42, 2515. (in Chinese) |
| [2] | 孙奇, 孙泽颖, 俞泽, 王光伟, 有机化学, 2022, 42, 2515). |
| [3] | (a) Hagan, D. O.; Wang, Y.; Skibinski, M.; Slawin, A. M. Z. Pure Appl. Chem. 2012, 84, 1587. |
| [3] | (b) Hu, J.; Zhang, W.; Wang, F. Chem. Commun. 2009, 7465. |
| [3] | (c) Ritter, S. K. Chem. Eng. News 2012, 90, 10. |
| [3] | (d) Ritter, S. K. Chem. Eng. News 2013, 91, 32. |
| [3] | (e) Li, Z.-Q.; Wang, C.-Q.; Feng, C. Chin. J. Org. Chem. 2022, 42, 3906. (in Chinese) |
| [3] | 李志强, 王成强, 冯超, 有机化学, 2022, 42, 3906). |
| [4] | (a) Prakash, G. K. S.; Zibinsky, M.; Upton, T. G.; Kashe-mirov, B. A.; McKenna, C. E.; Oertell, K.; Goodman, M. F.; Batra, V. K.; Pedersen, L. C.; Beard, W. A.; Shock, D. D.; Wilson, S. H.; Olah, G. A. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 15693. |
| [4] | (b) Hirai, G.; Watanabe, T.; Yamaguchi, K.; Miyagi, T.; Sodeoka, M. J. Am. Chem. Soc. 2007, 129, 15420. |
| [5] | (a) Bach, R. D.; Dmitrenko, O. J. Am. Chem. Soc. 2004, 126, 4444. |
| [5] | (b) Greenberg, A.; Tomkins, R. P. T.; Dobrovolny, M.; Liebman, J. F. J. Am. Chem. Soc. 1983, 105, 6855. |
| [6] | (a) Carter, F. L.; Frampton, V. L. Chem. Rev. 1964, 64, 497. |
| [6] | (b) Zhu, Z. B.; Wei, Y.; Shi, M. Chem. Soc. Rev. 2011, 40, 5534. |
| [6] | (c) Li, P.; Zhang, X.; Shi, M. Chem. Commun. 2020, 56, 5457. |
| [6] | (d) Song, C.; Wang, J.; Xu, Z. Acta Chim. Sinica 2015, 73, 1114. (in Chinese) |
| [6] | (宋传玲, 王建武, 徐政虎, 化学学报, 2015, 73, 1114.) |
| [6] | (e) Ravasco, J. M. J. M.; Monteiro, C.; Trindade, A. Org. Chem. Front. 2017, 4, 1167. |
| [6] | (f) Komatsu, K.; Kitagawa, T. Chem. Rev. 2003, 103, 1371. |
| [6] | (g) Raiguru, B. P.; Nayak, S.; Mishra, D. R.; Das, T.; Mohapatra, S.; Mishra, N. P. Asian J. Org. Chem. 2020, 9, 1088. |
| [7] | (a) Zhou, G.; Shen, X. Angew. Chem. Int. Ed. 2022, 61, e2021153. |
| [7] | (b) Zhang, Y.; Zhou, G.; Gong, X.; Guo, Z.; Qi, X.; Shen, X. Angew. Chem. Int. Ed. 2022, 61, e2022021. |
| [7] | (c) Myronova, V.; Cahard, D.; Marek, I. Org. Lett. 2022, 24, 9076. |
| [7] | (d) Morandi, B.; Carreira, E. M. Angew. Chem. Int. Ed. 2010, 49, 4294. |
| [8] | (a) Dolbier, W. R.; Battiste, M. A. Chem. Rev. 2003, 103, 1071. |
| [8] | (b) Fedoryn′ski, M. Chem. Rev. 2003, 103, 1099. |
| [8] | (c) Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827. |
| [8] | (d) Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140. |
| [9] | Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013. |
| [10] | Brisdon, A. K.; Crossley, I. R.; Flower, K. R.; Pritchard, R. G.; Warren, J. E. Angew. Chem. Int. Ed. 2003, 42, 2399. |
| [11] | Cheng, Z.-L.; Chen, Q.-Y. Chin. J. Chem. 2006, 24, 1219. |
| [12] | Hang, X.-C.; Gu, W.-P.; Chen, Q.-Y.; Xiao, J.-C. Tetrahedron 2009, 65, 6320. |
| [13] | Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem. Int. Ed. 2011, 50, 7153. |
| [14] | Nosik, P. S.; Pashko, M. O.; Poturai, A. S.; Kvasha, D. A.; Pashenko, A. E.; Rozhenko, A. B.; Suikov, S.; Volochnyuk, D. M.; Ryabukhin, S. V.; Yagupolskii, Y. L. Eur. J. Org. Chem. 2021, 6604. |
| [15] | Maruno, K.; Niina, K.; Nagata, O.; Shibata, N. Org. Lett. 2022, 24, 1722. |
| [16] | Rulliere, P.; Cyr, P.; Charette, A. B. Org. Lett. 2016, 18, 1988. |
| [17] | Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411. |
| [18] | Deng, X.-Y.; Lin, J.-H.; Zheng, J.; Xiao, J.-C. Chem. Commun. 2015, 51, 8805. |
| [19] | (a) Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624. |
| [19] | (b) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem. Int. Ed. 2009, 48, 9792. |
| [19] | (c) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. |
| [19] | (d) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. |
| [19] | (e) Wencel-Delord, J.; Dro?ge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740. |
| [19] | (f) Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H. Chem. Rev. 2012, 112, 5879. |
| [19] | (g) Huang, J.; Liu, F.; Zeng, L.-H.; Li, S.; Chen, Z.; Wu, J. Nat. Commun. 2022, 13, 7081. |
| [19] | (h) Kuhl, N.; Hopkinson, M. N.; Wencel- Delord, J.; Glorius, F. Angew. Chem. Int. Ed. 2012, 51, 10236. |
| [19] | (i) Zhou, K.; Huang, J.; Wu, J.; Qiu, G. Chin. Chem. Lett. 2021, 32, 37. |
| [19] | (j) He, F.-S.; Yang, M.; Ye, S.; Wu, J. Chin. Chem. Lett. 2021, 32, 461. |
| [19] | (k) Wang, X.; Zhang, J.; Chen, Q.; Zhou, W.; Wu, J. Chin. Chem. Lett. 2022, 33, 4860. |
| [19] | (l) Liu, Y.; Wang, L.; Zeng, L.-H.; Zhao, Y.; Zhu, T.; Wu, J. Chin. Chem. Lett. 2022, 33, 2383. |
| [20] | (a) Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007. |
| [20] | (b) Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H. Chem. Rev. 2012, 112, 5879. |
| [20] | (c) Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651. |
| [20] | (d) Xiao, W.; Wang, X.; Liu, R.; Wu, J. Chin. Chem. Lett. 2021, 32, 1847. |
| [21] | Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007. |
| [22] | Xu, H.; Chen, W.; Bian, M.; Xu, H.; Gao, H.; Wang, T.; Zhou, Z.; Yi, W. ACS Catal. 2021, 11, 14694. |
| [23] | He, Y.; Tian, L.; Chang, X.; Qu, Z.; Huang, Y.; Huang, C.; Sun, Q.; Wang, H. Chin. Chem. Lett. 2022, 33, 2987. |
| [24] | Liu, X.; Chen, J.; Yang, C.; Wu, Z.; Li, Z.; Shi, Y.; Huang, T.; Yang, Z.; Wu, Y. Org. Lett. 2021, 23, 6831. |
| [25] | Shen, M.; Li, H.; Zhang, X.; Fan, X. Org. Chem. Front. 2022, 9, 5976. |
| [26] | Li, Q.; Yan, K.; Zhu, Y.; Qi, G.; Wang, Y.; Hao, W.-J.; Jiang, B. Chin. Chem. Lett. 2023, DOI: 10.1016/j.cclet.2022.108014. |
| [27] | Wang, T.; Chen, J.; Shi, Y.-S.; Liu, X.-X.; Guo, L.; Wu, Y. Tetrahedron Lett. 2022, 99, 153845. |
| [28] | Zhang, Z.; Gevorgyan, V. J. Am. Chem. Soc. 2022, 144, 20875. |
| [29] | Nechaev, I. V.; Cherkaev, G. V.; Boev, N. V.; Solyev, P. N. J. Org. Chem. 2021, 86, 1037. |
| [30] | (a) Nechaev, I. V.; Cherkaev, G. V.; Solyev, P. N.; Boev, N. V. J. Org. Chem. 2021, 86, 4220. |
| [30] | (b) Nechaev, I. V.; Cherkaev, G. V. J. Org. Chem. 2021, 86, 7687. |
| [30] | (c) Nechaev, I. V.; Cherkaev, G. V.; Sheremetev, A. B. J. Org. Chem. 2022, 87, 652. |
| [31] | (a) Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235. |
| [31] | (b) Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295. |
| [31] | (c) Kumar, K. ChemistrySelect 2020, 5, 10298. |
| [31] | (d) Mathiyazhagan, A. D.; Anilkumar, G. Org. Biomol. Chem. 2019, 17, 6735. |
| [31] | (e) Huang, J.; Ding, F.; Rojsitthisak, P.; He, F.-S.; Wu., J. Org. Chem. Front. 2020, 7, 2873. |
| [31] | (f) Buyck, T.; Wang, Q.; Zhu, J. J. Am. Chem. Soc. 2014, 136, 11524. |
| [32] | (a) Du, J.; Xu, X.; Li, Y.; Pan. L.; Liu, Q. Org. Lett. 2014, 16, 4004. |
| [32] | (b) Dong, J.; Bao, L.; Hu, Z.; Ma, S.; Zhou, X.; Hao, M.; Li, N.; Xu, X. Org. Lett. 2018, 20, 1244. |
| [32] | (c) Kok, G. P. Y.; Yang, H.; Wong, M. W.; Zhao, Y. Org. Lett. 2018, 20, 5112. |
| [32] | (d) Bhattacharyya, A.; Shahi, C. K.; Pradhan, S.; Ghorai, M. K. Org. Lett. 2018, 20, 2925. |
| [33] | Dong, J.; Feng, W.; Wang, L.; Li, M.; Chen, Z.; Xu, X. Chem. Commun. 2021, 57, 12635. |
| [34] | Tang, X.; Liu, K.; Qu, Z.; Zhan, J.; Zhu, R.; Teng, F.; Meng, L.; Huang, Y.; Huang, C.; He, Y.; Zhu, Q. Chin. Chem. Lett. 2022, 33, 2982. |
| [35] | Xia, Y.; Qiu, D.; Wang, J. Chem. Rev. 2017, 117, 13810. |
| [36] | Tran, G.; Pardo, D. G.; Tsuchiya, T.; Hillebrand, S.; Vors, J.-P.; Cossy, J. Org. Lett. 2015, 17, 3414. |
| [37] | Muriel, B.; Gagnebin, A.; Waser, J. Chem. Sci. 2019, 10, 10716. |
| [38] | Huang, J.; Liu, F.; Wu, X.; Chen, J.-Q.; Wu, J. Org. Chem. Front. 2022, 9, 2840. |
| [39] | Xu, H.; Fang, X.-J.; Huang, W.-S.; Xu, Z.; Li, L.; Ye, F.; Cao, J.; Xu, L-W. Org. Chem. Front. 2022, 9, 5272. |
| [40] | Wang, Z.; Silverman, R. B. Bioorg. Med. Chem. 2006, 14, 2242. |
| [41] | Shibuya, A.; Sato, A.; Taguchi, T. Bioorg. Med. Chem. Lett. 1998, 8, 1979. |
| [42] | Staehle, W.; Schultz, M.; Schiemann, K. WO 2013/020622 A1, 2013 [Chem. Abstr. 2013, 158, 331209 ] |
| [43] | Backfisch, G.; Bakker, M.; Blaich, G.; Braje, W.; Drescher, K.; Erhard, T.; Haupt, A.; Hoft, C.; Kling, A.; Lakics, V.; Mack, H.; Oellien, F.; Peter, R.; Pohlki, F.; Relo, A. L. WO 2017/050807 A1, 2017 [Chem. Abstr 2017, 166, 384945 ] |
| [44] | Pfister, J. R.; Makra, F.; Muehldorf, A. V.; Wu, H.; Nelson, J. T.; Cheung, P.; Bruno, N. A.; Casey, S. M.; Zutshi, N.; Slate, D. L. Bioorg. Med. Chem. Lett. 1995, 5, 2473. |
| [45] | Ernouf, G.; Brayer, J.-L.; Folleas, B.; Demoute, J.-P.; Meyer, C.; Cossy, J. J. Org. Chem. 2017, 82, 3965. |
| [46] | Yamani, K.; Pierre, H.; Archambeau, A.; Meyer, C.; Cossy, J. Angew. Chem. Int. Ed. 2020, 59, 18505. |
| [47] | Li, X.; Li, L.; Tang, Y.; Zhong, L.; Cun, L.; Zhu, J.; Liao, J.; Deng, J. J. Org. Chem. 2010, 75, 2981. |
| [48] | Sekine, K.; Ushiyama, A.; Endo, Y.; Mikami, K. J. Org. Chem. 2020, 85, 7916. |
| [49] | Zhao, X.; Xu, S.; He, J.; Zhou, Y.; Cao, S. Org. Chem. Front. 2019, 6, 2539. |
| [50] | (a) Oestreich, M.; Hartmann, E.; Mewald, M. Chem. Rev. 2013, 113, 402. |
| [50] | (b) Tian, B.; Liu, Q.; Tian, P.; Lin, G.-Q. Org. Chem. Front. 2014, 1, 1116. |
| [50] | (c) Mun, S.-Y.; Lee, J.-E.; Yun, J. Org. Lett. 2006, 8, 4887. |
| [50] | (d) Zhang, J.; Dai, W.-P.; Cao, S. Org. Lett. 2017, 19, 3283. |
| [50] | (e) Kleeberg, C.; Feldmann, E.; Oestreich, M. Chem.-Eur. J. 2011, 17, 13538. |
| [51] | Sekine, K.; Akaishi, D.; Konagaya, K.; Ito, S. Chem. Eur. J. 2022, 28, e202200657. |
| [52] | (a) Ismail, F. M. D. J. Fluorine Chem. 2002, 118, 27. |
| [52] | (b) Bo?hm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Mu?ller, K.; ObstSander, U.; Stahl, M. ChemBioChem 2004, 5, 637. |
| [52] | (c) Isanobor, C.; O’Hagan, D. J. Fluorine Chem. 2006, 127, 303. |
| [52] | (d) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. |
| [52] | (e) Mu?ller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [52] | (f) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [53] | (a) Holmgren, S. K.; Taylor, K. M.; Bretscher, L. E.; Raines, R. T. Nature 1998, 392, 666. |
| [53] | (b) Bretscher, L. E.; Jenkins, C. L.; Taylor, K. M.; DeRider, M. L.; Raines, R. T. J. Am. Chem. Soc. 2001, 123, 777. |
| [53] | (c) Hodges, J. A.; Raines, R. T. J. Am. Chem. Soc. 2003, 125, 9262. |
| [53] | (d) Hodges, J. A.; Raines, R. T. J. Am. Chem. Soc. 2005, 127, 15923. |
| [54] | Smith, A. M. R.; Mimi) Hii, K. K. Chem. Rev. 2011, 111, 1637. |
| [55] | Li, L.; Ni, C.; Wang, F.; Hu, J. Nat. Commun. 2016, 7, 13320. |
| [56] | Wang, X.; Wang, F.; Huang, F.; Ni, C.; Hu, J. Org. Lett. 2021, 23, 1764. |
/
| 〈 |
|
〉 |