

金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★
收稿日期: 2023-03-30
网络出版日期: 2023-05-09
基金资助
国家自然科学基金(21821002); 国家自然科学基金(22031012); 国家自然科学基金(22071260)
Gold/Iridium Catalyzed Alkynylamide Cyclization/Asymmetric Allylic Benzylation Cascade Reaction★
Received date: 2023-03-30
Online published: 2023-05-09
Supported by
National Natural Science Foundation of China(21821002); National Natural Science Foundation of China(22031012); National Natural Science Foundation of China(22071260)
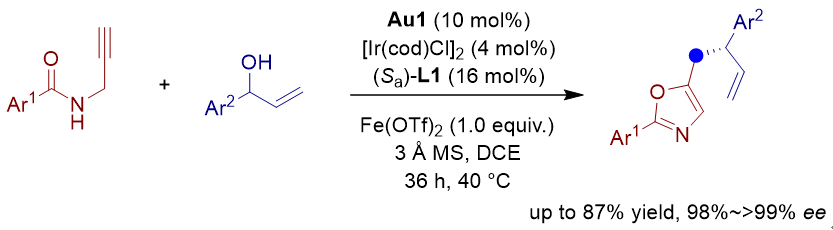
苄基亲核试剂参与的不对称烯丙基取代反应可以快速构筑含苄基片段的手性分子, 受到了有机化学家的广泛关注. 其中使用5-亚甲基二氢噁唑作为苄基亲核试剂等同体, 可以实现形式上的不对称苄位烯丙基取代反应. 然而由于5-亚甲基二氢噁唑化合物稳定性较差, 合成也存在一定困难, 对于发展高效的不对称苄位烯丙基取代反应提出了新挑战. 本工作发展了金/铱催化的炔基酰胺环化/不对称烯丙基苄基化串联反应. 首先金催化炔基酰胺环化生成5-亚甲基二氢噁唑, 随后亲核进攻烯丙基铱中间体, 以良好的收率(49%~87%)以及优秀的对映选择性控制(98%~>99% ee)得到芳基苄位烯丙基取代的手性分子.
王瑞祥 , 赵庆如 , 顾庆 , 游书力 . 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023 , 81(5) : 431 -434 . DOI: 10.6023/A23030103
Asymmetric allylic substitution reactions involving benzyl nucleophilic reagents can rapidly construct chiral molecules containing benzyl fragments, which has attracted widespread attention from organic chemists. Formal asymmetric allylic benzylation reactions have been achieved by utilizing methylene oxazole as an equivalent of benzyl nucleophile. However, the development of highly efficient asymmetric allylic benzylation reactions remains a great challenge mainly due to the poor stability and synthetic difficulty of methylene oxazole. In this work, we have developed gold- and iridium- catalyzed alkynylamide cyclization/asymmetric allylic benzylation cascade reactions. In the presence of gold-carbene complex (Au1) and the combination of [Ir(cod)Cl]2 and (Sa)-Carreira ligand, a wide range of enantioenriched oxazole derivatives, bearing a benzylic stereogenic center, were obtained in 49%~87% yields with 98%~>99% ee. A general procedure is described as the following: To a dried Schlenk tube were added [Ir(cod)Cl]2 (5.4 mg, 0.008 mmol, 4 mol%), (Sa)-L1 (16.2 mg, 0.032 mmol, 16 mol%) and 1,2-dichloroethane (1 mL) under argon atmosphere. The mixture was stirred at room temperature for 15 minutes to give a chiral iridium complex solution. Under an argon atmosphere, alkynylamide (0.2 mmol, 1.0 equiv.), allyl alcohol (0.4 mmol, 2.0 equiv.), Au1 (0.02 mmol, 12.4 mg, 10 mol%), Fe(OTf)2 (0.2 mmol, 70.6 mg, 1.0 equiv.) and 3 Å molecular sieves (80.0 mg) were added to another dry Schlenk tube, and then the above-prepared iridium catalyst was added. The reaction mixture was stirred at 40 ℃ until the starting materials were consumed (monitored by thin layer chromatography, TLC). The mixture was quenched with water (5 mL), and extracted with dichloromethane (5 mL×3). The combined organic layers were dried over anhydrous Na2SO4, filtered, and then concentrated in vacuo to afford the crude product. The residue was purified by column chromatography (V(petroleum ether)/V(ethyl acetate)=15/1 or 10/1) to afford product 3.

| [1] | (a) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921. |
| [1] | (b) Lu, Z.; Ma, S. Angew. Chem. Int. Ed. 2008, 47, 258. |
| [1] | (c) Weaver, J. D.; Recio, A., III; Grenning, A. J.; Tunge, J. A. Chem. Rev. 2011, 111, 1846. |
| [1] | For a book, see: |
| [1] | (d) Transition Metal Catalyzed Enantioselective Allylic Substitution: Organic Synthesis, Ed.: Kazmaier, U., Springer,eidelberg, 2012. |
| [2] | (a) Heterocycles in Natural Product Synthesis, Eds.: Majumdar, K. C.; Chattopadhyay, S. K., Wiley-VCH,Weinheim, 2011. |
| [2] | (b) Metalation of Azoles and Related Five-Membered Ring Heterocycles: Topics in Heterocyclic Chemistry, Vol. 29, Ed.: Gribble, G. W., Springer, Heidelberg, 2012. |
| [2] | (c) Heteroocyclic Chemistry in Drug Discovery, Ed.: Li, J.-J., Wiley, Hoboken, 2013. |
| [2] | (d) Roughley, S. D.; Jordan, A. M. J. Med. Chem. 2011, 54, 3451. |
| [2] | (e) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257. |
| [3] | (a) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2008, 130, 14092. |
| [3] | (b) Trost, B. M.; Thaisrivongs, D. A. J. Am. Chem. Soc. 2009, 131, 12056. |
| [3] | (c) Trost, B. M.; Thaisrivongs, D. A.; Hartwig, J. J. Am. Chem. Soc. 2011, 133, 12439. |
| [4] | (a) Sha, S.-C.; Jiang, H.; Mao, J.; Bellomo, A.; Jeong, S. A.; Walsh, P. J. Angew. Chem. Int. Ed. 2016, 55, 1070. |
| [4] | (b) Mao, J.; Zhang, J.; Jiang, H.; Bellomo, A.; Zhang, M.; Gao, Z.; Dreher, S. D.; Walsh, P. J. Angew. Chem. Int. Ed. 2016, 55, 2526. |
| [5] | (a) Mosrin, M.; Knochel, P. Org. Lett. 2009, 11, 1837. |
| [5] | (b) Duez, S.; Steib, A. K.; Manolikakes, S. M.; Knochel, P. Angew. Chem. Int. Ed. 2011, 50, 7686. |
| [5] | (c) Haas, D.; Hammann, J. M.; Greiner, R.; Knochel, P. ACS Catal. 2016, 6, 1540. |
| [6] | (a) Liu, X.-J.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 4002. |
| [6] | (b) Liu, X.-J.; Zhang, W.-Y.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, e202200164. |
| [7] | For selected reviews on Ir-catalyzed asymmetric allylic substitution reactions, see: a Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461. |
| [7] | (b) Liu, W.-B.; Xia, J.-B.; You, S.-L. Top. Organomet. Chem. 2012, 38, 155. |
| [7] | (c) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558. |
| [7] | (d) Hethcox, J. C.; Shockley, S. E.; Stoltz, B. M. ACS Catal. 2016, 6, 6207. |
| [7] | (e) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539. |
| [7] | (f) R?ssler, S. L.; Petrone, D. A.; Carreira, E. M. Acc. Chem. Res. 2019, 52, 2657. |
| [7] | (g) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855. |
| [8] | (a) Singha, S.; Serrano, E.; Mondal, S.; Daniliuc, C. G.; Glorius, F. Nat. Catal. 2020, 3, 48. |
| [8] | (b) Butcher, T. W.; Yang, J. L.; Amberg, W. M.; Watkins, N. B.; Wilkinson, N. D.; Hartwig, J. F. Nature 2020, 583, 548. |
| [8] | (c) Chen, P.; Li, Y.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Angew. Chem. Int. Ed. 2020, 59, 7083. |
| [8] | (d) Han, M.; Yang, M.; Wu, R.; Li, Y.; Jia, T.; Gao, Y.; Ni, H.-L.; Hu, P.; Wang, B.-Q.; Cao, P. J. Am. Chem. Soc. 2020, 142, 13398. |
| [8] | (e) Tu, H.-F.; Yang, P.; Lin, Z.; Zheng, C.; You, S.-L. Nat. Chem. 2020, 12, 838. |
| [8] | (f) Rossi-Ashton, J. A.; Clarke, A. K.; Donald, J. R.; Zheng, C.; Taylor, R. J. K.; Unsworth, W. P.; You, S.-L. Angew. Chem. Int. Ed. 2020, 59, 7598. |
| [8] | (g) Wang, J.; Qi, X.; Min, X.-L.; Yi, W.; Liu, P.; He, Y. J. Am. Chem. Soc. 2021, 143, 10686. |
| [8] | (h) Davis, C. R.; Luvaga, I. K.; Ready, J. M.; J. Am. Chem. Soc. 2021, 143, 4921. |
| [8] | (i) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380. |
| [8] | (j) Crisenza, G. E. M.; Faraone, A.; Gandolfo, E.; Mazzarella, D.; Melchiorre, P. Nat. Chem. 2021, 13, 575. |
| [8] | (k) Xiao, L.; Wei, L.; Wang, C.-J. Angew. Chem. Int. Ed. 2021, 60, 24930. |
| [8] | (l) Peng, Y.; Huo, X.; Luo, Y.; Wu, L.; Zhang, W. Angew. Chem. Int. Ed. 2021, 60, 24941. |
| [8] | (m) Zhang, M.-M.; Chen, P.; Xiong, W.; Hui, X.-S.; Lu, L.-Q.; Xiao, W.-J. CCS Chem. 2021, 3, 3383. |
| [8] | (n) Deng, Y.; Liang, X.; Wei, K.; Yang, Y.-R. J. Am. Chem. Soc. 2021, 143, 20622. |
| [8] | (o) Zhao, Q.-R.; Jiang, R.; You, S.-L. Acta Chim. Sinica 2021, 79, 1107. (in Chinese) |
| [8] | (赵庆如, 蒋茹, 游书力, 化学学报, 2021, 79, 1107.) |
| [8] | (p) Yang, P.-S.; Liu, C.-X.; Zhang, W.-W.; You, S.-L. Acta Chim. Sinica 2021, 79, 742. (in Chinese) |
| [8] | (杨普苏, 刘晨旭, 张文文, 游书力, 化学学报, 2021, 79, 742.) |
| [8] | (q) Ding, L.; Song, H.; Zheng, C.; You, S.-L. J. Am. Chem. Soc. 2022, 144, 4770. |
| [8] | (r) Liu, X.-J.; Zhang, W.-Y.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, 61, e2022001. |
| [8] | (s) Moghadam, F. A.; Hicks, E. F.; Sercel, Z. P.; Cusumano, A. Q.; Bartberger, M. D.; Stoltz, B. M. J. Am. Chem. Soc. 2022, 144, 7983. |
| [8] | (t) Yang, P.; Wang, R.-X.; Cheng, Y.-Z.; Zheng, C.; You, S.-L. Angew. Chem. Int. Ed. 2022, 61, e202213520. |
| [8] | (u) Yang, W.-L.; Shang, X.-Y.; Luo, X.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202203661. |
| [8] | (v) Jiang, R.; Zhao, Q.-R.; Zheng, C.; You, S.-L. Nat. Catal. 2022, 5, 1089. |
| [8] | (w) Zhang, J.; Yang, W.-L.; Zheng, H.; Wang, Y.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202117079. |
| [8] | (x) Wu, Z.-H.; Wang, H.-Y.; Yang, H.-L.; Wei, L.-H.; Hayashi, T.; Duan, W.-L. Angew. Chem. Int. Ed. 2022, 61, e202213904. |
| [8] | (y) Yang, W.-L.; Shang, X.-Y.; Ni, T.; Yan, H.; Luo, X.; Zhen, H.; Li, Z.; Deng, W.-P. Angew. Chem. Int. Ed. 2022, 61, e202210207. |
| [8] | (z) Fu, C.; Xiong, Q.; Xiao, L.; He, L.; Bai, T.; Zhang, Z.; Dong, X.-Q.; Wang, C.-J. Chin. J. Chem. 2022, 40, 1059. |
| [8] | (aa) Tang, X.; Su, Z.; Lin, Q.; Lin, L.; Dong, S.; Feng, X. Chin. J. Chem. 2022, 40, 1793. |
| [8] | (ab) Jia, S.-H.; Chen, S.-Y.; Liu, Z.-S.; Cheng, H.-G.; Zhou, Q.-H. Chin. J. Org. Chem. 2022, 42, 3373. (in Chinese) |
| [8] | (贾仕虎, 陈思元, 刘泽水, 程鸿刚, 周强辉, 有机化学, 2022, 42, 3373.) |
| [8] | (ac) Xie, J.-H.; Hou, Y.-M.; Feng, Z.; You, S.-L. Angew. Chem. Int. Ed. 2023, 63, e202216396. |
| [9] | Liu, X.-J.; Zheng, C.; Yang, Y.-H.; Jin, S.; You, S.-L. Angew. Chem. Int. Ed. 2019, 58, 10493. |
| [10] | (a) Seppanen, O.; Aikonen, S.; Muuronen, M.; Alamillo-Ferrer, C.; Bures, J.; Helaja, J. Chem. Commun. 2020, 56, 14697. |
| [10] | (b) Yang, G.; Ke, Y.-M.; Zhao., Y. Angew. Chem. Int. Ed. 2021, 60, 12775. |
| [10] | (c) Ma, Y.; Ali, H. S.; Hussein, A. A. Catal. Sci. Technol. 2022, 12, 674. |
| [11] | (a) Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S. Chem. Rev. 2020, 120, 13382. |
| [11] | (b) Wei, L.; Wang, C.-J. Chem Catal. 2023, 3, 100455. |
/
| 〈 |
|
〉 |