

离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极
收稿日期: 2023-05-10
网络出版日期: 2023-07-25
基金资助
国家自然科学基金(22179018); 国家自然科学基金(22279028); 辽宁省自然科学基金(2021MS132); 山东省自然科学基金(ZR2020QB129); 河北省杰出青年基金(B2021205019)
The Preparation of Carbon Nanotubes/Reduced Graphene Oxide Current Collector by Non-covalent Induction of Ionic Liquid for Sodium Metal Anode
Received date: 2023-05-10
Online published: 2023-07-25
Supported by
National Natural Science Foundation of China(22179018); National Natural Science Foundation of China(22279028); Natural Science Foundation of Liaoning Province(2021MS132); Shandong Provincial Natural Science Foundation(ZR2020QB129); Natural Science Foundation of Hebei Province(B2021205019)
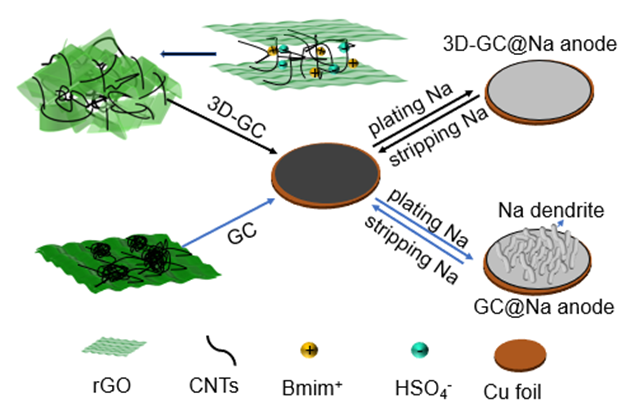
钠金属电池因丰富的钠资源储备在大规模储能中具有广泛的应用前景. 然而钠金属电池存在钠金属活性高、形成的固体电解质界面膜(SEI膜)不稳定、金属钠体积膨胀大等问题, 限制了其应用. 本工作利用离子液体与sp2碳间的非共价作用, 诱导碳纳米管和还原氧化石墨烯进行自组装, 制备得到了碳纳米管支撑的石墨烯高导电三维多孔碳(3D-GC)集流体. 制备得到的3D-GC具有三维多孔结构, 可以为金属钠提供较大的储存空间. 另外, 复合材料具有较高的导电性, 得到了较低的金属钠的沉积过电位(5.6 mV). 3D-GC@Na负极具有良好的电化学性能, 在电流密度为1 mA•cm−2, 沉积容量为1 mAh•cm−2时, 在240次充放电循环过程中始终保持了较高的库伦效率(CE), 平均CE为99.6%. 与Na3V2(PO4)2F3组装全电池也表现出优异的电化学性能.
刘稳 , 王昱捷 , 杨慧琴 , 李成杰 , 吴娜 , 颜洋 . 离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极[J]. 化学学报, 2023 , 81(10) : 1379 -1386 . DOI: 10.6023/A23050220
Sodium metal batteries have been regarded as promising candidates for next-generation energy storage systems due to their impressive capacity and natural abundance. However, the high reactivity of Na, unstable solid electrolyte interface (SEI) and Na metal dendrite growth with safety hazards inhibit their applications. Various strategies have been proposed to solve the above issues. Designing porous current collectors has been recognized as one of the most promising solutions. Porous carbon/carbon nanotubes/graphene- based materials are widely investigated as host materials for sodium metal anode. However, the sp2 carbon faces serious issues, such as aggregation or stacking because of their π-π interactions. Herein, we tackle this issue by using ionic liquid as additive during hydrothermal process. The non-covalent interaction between 1-butyl-3-methylimidazolium (Bmim+) and sp2 carbon (carbon nanotubes and reduced graphene oxide) helps to inhibit the aggregation of CNTs and the stacking of rGO layers. Also, their interactions induced the CNTs and rGO to form three dimensional (3D) porous carbon (3D-GC) current collector. The ionic liquid 1-butyl-3-methylimidazolium bisulfate ([Bmim][HSO4]) plays great role as a stabilizer and surfactant. The reduced surface tension of the system is also favorable for uniformly interweaving the CNTs and rGO. The prepared 3D-GC exhibit micro-meso-macro porous structure, which provides a large storage space for sodium metal. Meantime, the composite shows a high electrical conductivity, leading to a low deposition overpotential (5.6 mV) of sodium metal. As a result, the 3D-GC@Na anode exhibit an impressive cycling stability for over 1450 cycles (2900 h) at 1 mA•cm−2 with a capacity of 1 mAh•cm−2. Moreover, when being used in full cells with Na3V2(PO4)2F3 as cathode, they also show well performances.

| [1] | Wahid, M.; Puthusseri, D.; Gawli, Y.; Sharma, N.; Ogale, S. ChemSusChem 2018, 11, 506. |
| [2] | Kang, S. S.; Fan, S. C.; Liu, Y.; Wei, Y. C.; Li, Y.; Fang, J. G.; Meng, C. Z. Acta Chim. Sinica 2019, 77, 647 (in Chinese). |
| [2] | (康树森, 范少聪, 刘岩, 魏彦存, 李营, 房金刚, 孟垂舟, 化学学报, 2019, 77, 647.) |
| [3] | Liang, S. S.; Kang, S. S.; Yang, D.; Hu, J. H. Acta Chim. Sinica 2022, 80, 1264 (in Chinese). |
| [3] | (梁世硕, 康树森, 杨东, 胡建华, 化学学报, 2022, 80, 1264.) |
| [4] | Chen, Q. L.; Liu, B.; Zhang, L.; Xie, Q. S.; Zhang, Y. G.; Lin, J.; Qu, B. H.; Wang, L. S.; Sa, B. S.; Peng, D. L. Chem. Eng. J. 2021, 404, 126469. |
| [5] | Liu, Y.; Li, Q. Z.; Lei, Y. Y.; Zhou, D. L.; Wu, W. W.; Wu, X. H. J. Alloy. Compd. 2022, 926, 166850. |
| [6] | Shi, H.; Zhang, Y. M.; Liu, Y.; Yuan, C. Z. Chem. Rec. 2022, 22, 202200112. |
| [7] | Liu, T. F.; Yang, X. K.; Nai, J. W.; Wang, Y.; Liu, Y. J.; Liu, C. T.; Tao, X. Y. Chem. Eng. J. 2021, 409, 127943. |
| [8] | Wang, Y.; Wang, Y.; Wang, Y.-X.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Chem 2019, 5, 2547. |
| [9] | Xu, X. Y.; Li, Y. Y.; Cheng, J.; Hou, G. M.; Nie, X. K.; Ai, Q.; Dai, L. N.; Feng, J. K.; Ci, L. J. Energy Chem. 2020, 41, 73. |
| [10] | Xie, D.; Li, H. H.; Diao, W. Y.; Jiang, R.; Tao, F. Y.; Sun, H. Z.; Wu, X. L.; Zhang, J. P. Energy Stor. Mater. 2021, 36, 504. |
| [11] | Wu, W.; Hou, S.; Zhang, C.; Zhang, L. ACS Appl. Mater. Interfaces 2020, 12, 27300. |
| [12] | Eshetu, G. G.; Elia, G. A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Adv. Energy Mater. 2020, 10, 2000093. |
| [13] | Yan, J.; Zhi, G.; Kong, D. Z.; Wang, H.; Xu, T. T.; Zang, J. T.; Shen, W. X.; Xu, J. M.; Shi, Y. M.; Dai, S. G.; Li, X. J.; Wang, Y. J. Mater. Chem. A 2020, 8, 19843. |
| [14] | Zhao, C.; Lu, Y.; Yue, J.; Pan, D.; Qi, Y.; Hu, Y.-S.; Chen, L. J. Energy Chem. 2018, 27, 1584. |
| [15] | Ma, C. Y.; Xu, T. T.; Wang, Y. Energy Stor. Mater. 2020, 25, 811. |
| [16] | Miao, R. Q.; Wang, C. Z.; Li, D. L.; Sun, C.; Li, J. B.; Jin, H. B. Small 2022, 18, 2204487. |
| [17] | Fang, W.; Jiang, H.; Zheng, Y.; Zheng, H.; Liang, X.; Sun, Y.; Chen, C. H.; Xiang, H. F. J. Power Sources 2020, 455, 227956. |
| [18] | Seh, Z. W.; Sun, J.; Sun, Y. M.; Cui, Y. ACS Cent. Sci. 2015, 1, 449. |
| [19] | Zhao, L. F.; Hu, Z.; Huang, Z. Y.; Tao, Y.; Lai, W. H.; Zhao, A. L.; Liu, Q. N.; Peng, J.; Lei, Y. J.; Wang, Y. X.; Cao, Y. L.; Wu, C.; Chou, S. L.; Liu, H. K.; Dou, S. X. Adv. Energy Mater. 2022, 12, 2200990. |
| [20] | Zhu, M.; Wang, G. Y.; Liu, X.; Guo, B. K.; Xu, G.; Huang, Z. Y.; Wu, M.; Liu, H. K.; Dou, S. X.; Wu, C. Angew. Chem. Int. Ed. 2020, 59, 6596. |
| [21] | Zhang, Q.; Lu, Y.; Zhou, M.; Liang, J.; Tao, Z.; Chen, J. Inorg. Chem. Front. 2018, 5, 864. |
| [22] | Xiong, W. S.; Jiang, Y.; Xia, Y.; Qi, Y. Y.; Sun, W. W.; He, D.; Liu, Y. M.; Zhao, X. Z. Chem. Commun. 2018, 54, 9406. |
| [23] | Xu, Y. L.; Menon, A. S.; Harks, P. P. R. M. L.; Hermes, D. C.; Haverkate, L. A.; Unnikrishnan, S.; Mulder, F. M. Energy Stor. Mater. 2018, 12, 69. |
| [24] | Chu, C. X.; Wang, N. N.; Li, L. L.; Lin, L. D.; Tian, F.; Li, Y. L.; Yang, J.; Dou, S. X.; Qian, Y. T. Energy Stor. Mater. 2019, 23, 137. |
| [25] | Park, B.; Oh, S. M.; Jin, X.; Adpakpang, K.; Lee, N. S.; Hwang, S. J. Chem 2017, 23, 6544. |
| [26] | Liu, H. J.; Osenberg, M.; Ni, L.; Hilger, A.; Chen, L. B.; Zhou, D.; Dong, K.; Arlt, T.; Yao, X. Y.; Wang, X. G.; Manke, I.; Sun, F. J. Energy Chem. 2021, 61, 61. |
| [27] | Zhao, Y.; Yang, X. F.; Kuo, L. Y.; Kaghazchi, P.; Sun, Q.; Liang, J. N.; Wang, B. Q.; Lushington, A.; Li, R. Y.; Zhang, H. M.; Sun, X. L. Small 2018, 14, 1703717. |
| [28] | Zheng, Z.; Zeng, X. X.; Ye, H.; Cao, F. F.; Wang, Z. B. ACS Appl. Mater. Interfaces 2018, 10, 30417. |
| [29] | Sui, D.; Huang, Y.; Huang, L.; Zhang, Y.; Chen, Y. S. Acta Chim. Sinica 2014, 72, 382. |
| [30] | Olsson, E.; Chai, G.; Dove, M.; Cai, Q. Nanoscale 2019, 11, 5274. |
| [31] | Wang, A. X.; Hu, X. F.; Tang, H. Q.; Zhang, C. Y.; Liu, S.; Yang, Y. W.; Yang, Q. H.; Luo, J. Y. Angew Chem. Int. Ed. 2017, 56, 11921. |
| [32] | Zhao, Y.; Liu, H.; Kou, Y.; Li, M.; Zhu, Z.; Zhuang, Q. Electrochem. Commun. 2007, 9, 2457. |
| [33] | Yan, Y.; Li, P. Q.; Gu, Z. Y.; Liu, W.; Cao, J. M.; Wu, X. L. Chem. Eng. J. 2022, 432, 134195. |
| [34] | Zhang, Y.; Sun, J.; Liu, W.; Niu, Z. Y.; Yan, Y.; Qiao, L. Z.; Wu, N. Adv. Mater. Interfaces 2022, 9, 2200752. |
| [35] | Li, P. Q. M.S. Thesis, Dalian University of Technology, Dalian, 2022 (in Chinese). |
| [35] | ( 李培权, 硕士论文,大连理工大学,大连, 2022.) |
/
| 〈 |
|
〉 |