

草酸酯类化合物在自由基脱羟基化反应中的研究进展
收稿日期: 2023-07-15
网络出版日期: 2023-08-24
基金资助
国家自然科学基金(22201201); 浙江省自然科学基金(LY23B020001)
Recent Advances in Radical-Based Dehydroxylation of Hydroxyl Groups via Oxalates
Received date: 2023-07-15
Online published: 2023-08-24
Supported by
National Natural Science Foundation of China(22201201); Natural Science Foundation of Zhejiang Province(LY23B020001)
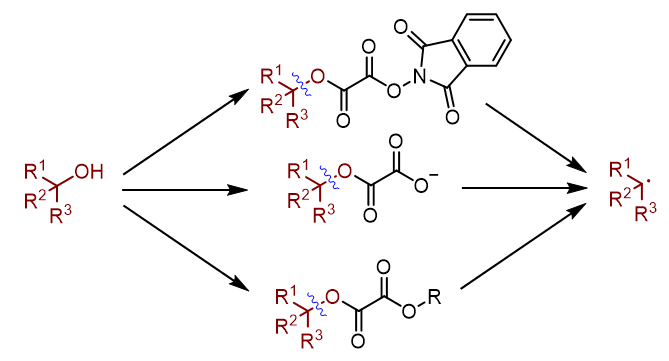
羟基作为醇类化合物的基本结构单元, 广泛存在于药物、天然产物、农药以及精细化学品中. 烷基自由基则是自由基化学领域最基础性的合成砌块. 因此, 将醇转化为烷基自由基, 具有基础性的研究价值. 通常醇类化合物可以通过Barton-McCombie反应, 实现自由基形式的脱羟基化反应, 得到烷基自由基. 然而传统脱羟基化反应存在诸多缺陷. 因此发展一种简洁高效的脱羟基化方法具有重要的现实意义. 随着近年来有机化学的发展, 自由基脱羟基化反应取得突破性进展. 本综述节选其中一部分, 着重介绍了草酸酯类化合物在自由基脱羟基化反应中的研究进展和设计原理, 对比不同活化策略的反应机理, 系统性地总结了邻苯二甲酰亚胺类型草酸酯、草酸单酯和草酸酯类化合物在自由基脱羟基化反应中的共性和个性, 展望了自由基脱羟基化反应的未来和趋势.
陈健强 , 朱钢国 , 吴劼 . 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023 , 81(11) : 1609 -1623 . DOI: 10.6023/A23070339
Alcohols are among the most synthetically versatile, operationally convenient, and commercially abundant functional groups in modern organic chemistry, which are widely present in a variety of drugs, natural products, agrochemicals, and fine chemicals. Alkyl radicals are the most important building blocks in radical chemistry. Traditionally, the alkyl radicals can be generated from the alcohols through the Barton-McCombie radical deoxygenation reaction. Despite the importance of this reaction, one of the several limitations of this strategy is the requirement of stoichiometric quantities of hazardous reagents. Another limitation is that the generated alkyl radical can abstract a hydrogen atom from tributyltin hydride to deliver an alkane. Therefore, development of a convenient and compatible method for generation of alkyl radicals from alcohols has synthetic appeal, yet it represents a long-term challenge and urgent demand. With the development of organic chemistry, much efforts have been devoted to the development of this transformation. This paper reviews the recent advances in dehydroxylation of hydroxyl groups via oxalates, focuses on the design principles and concepts of these strategies, compares the reaction mechanisms, and summarizes the generalities and differences of these methods. It is divided into three sections consisting of the radical-based dehydroxylation of hydroxyl groups via N‑phthalimidoyl oxalates, oxalate monoester salts and oxalates. The future and trend of radical-based dehydroxylation of hydroxyl groups in organic chemistry were prospected.

Key words: photocatalysis; free radicals; dehydroxylation; alcohols; oxalates
| [1] | (a) Ertl P.; Schuhmann T. J. Nat. Prod. 2019, 82, 1258. |
| [1] | (b) Henkel T.; Brunne R. M.; Müller H.; Reichel F. Angew. Chem. Int. Ed. 1999, 38, 643. |
| [2] | For reviews, see: (a) Hatwig W. Tetrahedron 1983, 39, 2609. |
| [2] | (b) Crich D.; Quintero L. Chem. Rev. 1989, 89, 1413. |
| [3] | (a) Zhang Z.; Gong L.; Zhou X.-Y.; Yan S.-S.; Li J.; Yu D.-G. Acta Chim. Sinica 2019, 77, 783.. (in Chinese) |
| [3] | ( 张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚, 化学学报, 2019, 77, 783.) |
| [3] | (b) Chen J.-Q.; Tu X.; Tang Q.; Li K.; Xu L.; Wang S.; Ji M.; Li Z.; Wu J. Nat. Commun. 2021, 12, 5328. |
| [3] | (c) Ji M.; Xu L.; Luo X.; Jiang M.; Wang S.; Chen J.; Wu J. Org. Chem. Front. 2021, 8, 6704. |
| [3] | (d) Yang M.; Ye B.; Chen J.; Wu J. Acta Chim. Sinica 2022, 80, 11.. (in Chinese) |
| [3] | ( 杨民, 叶柏柏, 陈健强, 吴劼, 化学学报, 2022, 80, 11.) |
| [3] | (e) Chen J.-Q.; Tu X.; Qin B.; Huang S.; Zhang J.; Wu J. Org. Lett. 2022, 24, 642. |
| [3] | (f) Chen J.-Q.; Chen Q.; Chen B.; Wu J. Org. Chem. Front. 2023, 10, 2018. |
| [3] | (g) Hou H.; Cheng Y.; Chen B.; Tung C.; Wu L. Chin. J. Org. Chem. 2023, 43, 1012.. (in Chinese) |
| [3] | ( 侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠, 有机化学, 2023, 43, 1012.) |
| [4] | (a) Zheng X.; Dai X.-J.; Yuan H.-Q.; Ye C.-X.; Ma J.; Huang P.-Q. Angew. Chem. Int. Ed. 2013, 52, 3494. |
| [4] | (b) Xie H.; Guo J.; Wang Y.-Q.; Wang K.; Guo P.; Su P.-F.; Wang X.; Shu X.-Z. J. Am. Chem. Soc. 2020, 142, 16787. |
| [4] | (c) Pang X.; Su P.-F.; Shu X.-Z. Acc. Chem. Res. 2022, 55, 2491. |
| [5] | (a) Nguyen J. D.; Matsuura B. S.; Stephenson C. R. J. J. Am. Chem. Soc. 2014, 136, 1218. |
| [5] | (b) Nacsa E. D.; MacMillan D. W. C. J. Am. Chem. Soc. 2018, 140, 3322. |
| [5] | (c) Zhao G.; Yao W.; Mauro J. N.; Ngai M.-Y. J. Am. Chem. Soc. 2021, 143, 1728. |
| [6] | (a) Stache E. E.; Ertel A. B.; Rovis T.; Doyle A. G. ACS Catal. 2018, 8, 11134. |
| [6] | (b) Hu X.-Q.; Hou Y.-X.; Liu Z.-K.; Gao Y. Org. Chem. Front. 2020, 7, 2319. |
| [6] | (c) Shao X.; Zheng Y.; Ramadoss V.; Tian L.; Wang Y. Org. Biomol. Chem. 2020, 18, 5994. |
| [6] | (d) Guo H.-M.; Wu X. Nat. Commun. 2021, 12, 5365. |
| [7] | (a) Dong Z.; MacMillan D. W. C. Nature 2021, 598, 451. |
| [7] | (b) Sakai H. A.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 6185. |
| [7] | (c) Intermaggio N. E.; Millet A.; Davis D. L.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 11961. |
| [8] | (a) Barton D. H. R.; Crich D. Tetrahedron Lett. 1985, 26, 757. |
| [8] | (b) Barton D. H. R.; Crich D.; Kretzschmar G. J. Chem. Soc., Perkin Trans. 1 1986, 39. |
| [9] | Lackner G. L.; Quasdorf K. W.; Overman L. E. J. Am. Chem. Soc. 2013, 135, 15342. |
| [10] | Lackner G. L.; Quasdorf K. W.; Pratsch G.; Overman L. E. J. Org. Chem. 2015, 80, 6012. |
| [11] | Gao C.; Li J.; Yu J.; Yang H.; Fu H. Chem. Commun. 2016, 52, 7292. |
| [12] | Chen X.; Luo X.; Peng X.; Guo J.; Zai J.; Wang P. Chem. Eur. J. 2020, 26, 3226. |
| [13] | (a) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2015, 137, 11270. |
| [13] | (b) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2016, 138, 1724. |
| [14] | Lowry M. S.; Goldsmith J. L.; Slinker J. D.; Rohl R.; Pascal R. A.; Malliaras G. G.; Bernhard S. Chem. Mater. 2005, 17, 5712. |
| [15] | Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Am. Chem. Soc. 2017, 139, 7192. |
| [16] | Garnsey M. R.; Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Org. Chem. 2018, 83, 6958. |
| [17] | Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. Angew. Chem. Int. Ed. 2020, 59, 6268. |
| [18] | Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. J. Org. Chem. 2020, 85, 15532. |
| [19] | Abbas S. Y.; Zhao P.; Overman L. E. Org. Lett. 2018, 20, 868. |
| [20] | Pitre S. P.; Muuronen M.; Fishman D. A.; Overman L. E. ACS Catal. 2019, 9, 3413. |
| [21] | Dong J.; Wang Z.; Wang X.; Song H.; Liu Y.; Wang Q. J. Org. Chem. 2019, 84, 7532. |
| [22] | Lipp B.; Nauth A. M.; Opatz T. J. Org. Chem. 2016, 81, 6875. |
| [23] | Iwai K.; Takemura F.; Furue M.; Nozakura S.-I. Bull. Chem. Soc. Jpn. 1984, 57, 763. |
| [24] | Zuo Z.; MacMillan D. W. C. J. Am. Chem. Soc. 2014, 136, 5257. |
| [25] | Amos S. G. E.; Cavalli D.; Vaillant F. L.; Waser J. Angew. Chem. Int. Ed. 2021, 60, 23827. |
| [26] | Li M.; Liu T.; Li J.; He H.; Dai H.; Xie J. J. Org. Chem. 2021, 86, 12386. |
| [27] | Luo J.; Zhang J. ACS Catal. 2016, 6, 873. |
| [28] | Zhang X.; MacMillan D. W. C. J. Am. Chem. Soc. 2016, 138, 13862. |
| [29] | Guo L.; Song F.; Zhu S.; Li H.; Chu L. Nat. Commun. 2018, 9, 4543. |
| [30] | (a) Wang X.; Chen Y.; Liang P.; Chen J.-Q.; Wu J. Org. Chem. Front. 2022, 9, 4328. |
| [30] | (b) Chen M.; Sun W.; Yang J.; Yuan L.; Chen J.; Wu J. Green Chem. 2023, 25, 3857. |
| [31] | Guo L.; Tu H.-Y.; Zhu S.; Chu L. Org. Lett. 2019, 21, 4771. |
| [32] | Li H.; Guo L.; Feng X.; Huo L.; Zhu S.; Chu L. Chem. Sci. 2020, 11, 4904. |
| [33] | Su J. Y.; Gru?nenfelder D. C.; Takeuchi K.; Reisman S. E. Org. Lett. 2018, 20, 4912. |
| [34] | Brioche J. Tetrahedron Lett. 2018, 59, 4387. |
| [35] | Gonzalez-Esguevillas M.; Miró J.; Jeffrey J. L.; MacMillan D. W. C. Tetrehedron 2019, 75, 4222. |
| [36] | Troyano J. A.; Ballaschk F.; Jaschinski M.; ?zkaya Y.; Gómez-Suárez A. Chem. Eur. J. 2019, 25, 14054. |
| [37] | Vincent é.; Brioche J. Eur. J. Org. Chem. 2021, 2421. |
| [38] | Wang Q.; Yue L.; Bao Y.; Wang Y.; Kang D.; Gao Y.; Yuan Z. J. Org. Chem. 2022, 87, 8237. |
| [39] | Yan X. B.; Li C.-L.; Jin W.-J.; Guo P.; Shu X.-Z. Chem. Sci. 2018, 9, 4529. |
| [40] | Ye Y.; Chen H.; Sessler J. L.; Gong H. J. Am. Chem. Soc. 2019, 141, 820. |
| [41] | Gao M.; Sun D.; Gong H. Org. Lett. 2019, 21, 1645. |
| [42] | Ye Y.; Chen H.; Yao K.; Gong H. Org. Lett. 2020, 22, 2070. |
| [43] | Chen H.; Ye Y.; Tong W.; Fang J.; Gong H. Chem. Commun. 2020, 56, 454. |
| [44] | Ye Y.; Ma G.; Yao K.; Gong H. Synlett 2021, 32, 1625. |
| [45] | Friese F. W.; Studer A. Angew. Chem. Int. Ed. 2019, 58, 9561. |
| [46] | Ma G.; Chen C.; Talukdar S.; Zhao X.; Lei C.; Gong H. Chem. Commun. 2020, 56, 10219. |
| [47] | Guo P.; Wang K.; Jin W.-J.; Xie H.; Qi L.; Liu X.-Y.; Shu X.-Z. J. Am. Chem. Soc. 2021, 143, 513. |
| [48] | Chen Y.; Wang F.; Liu B.-X.; Rao W.-D.; Wang S.-Y. Org. Chem. Front. 2022, 9, 731. |
| [49] | Zhuo J.; Zhu C.; Wu J.; Li Z.; Li C. J. Am. Chem. Soc. 2022, 144, 99. |
/
| 〈 |
|
〉 |