化学学报 ›› 2023, Vol. 81 ›› Issue (11): 1609-1623.DOI: 10.6023/A23070339 上一篇 下一篇
综述
投稿日期:2023-07-15
发布日期:2023-08-24
基金资助:
Jianqiang Chena,b, Gangguo Zhua( ), Jie Wub,c(
), Jie Wub,c( )
)
Received:2023-07-15
Published:2023-08-24
Contact:
*E-mail: Supported by:文章分享
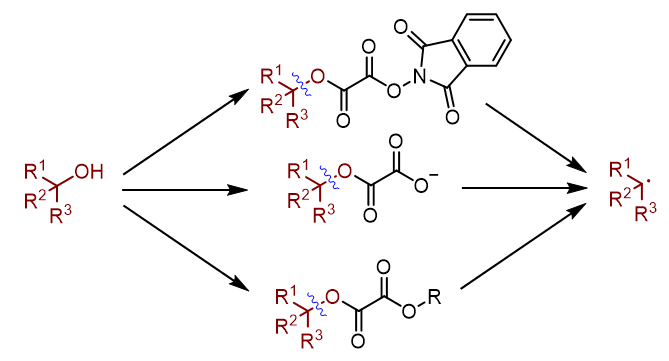
羟基作为醇类化合物的基本结构单元, 广泛存在于药物、天然产物、农药以及精细化学品中. 烷基自由基则是自由基化学领域最基础性的合成砌块. 因此, 将醇转化为烷基自由基, 具有基础性的研究价值. 通常醇类化合物可以通过Barton-McCombie反应, 实现自由基形式的脱羟基化反应, 得到烷基自由基. 然而传统脱羟基化反应存在诸多缺陷. 因此发展一种简洁高效的脱羟基化方法具有重要的现实意义. 随着近年来有机化学的发展, 自由基脱羟基化反应取得突破性进展. 本综述节选其中一部分, 着重介绍了草酸酯类化合物在自由基脱羟基化反应中的研究进展和设计原理, 对比不同活化策略的反应机理, 系统性地总结了邻苯二甲酰亚胺类型草酸酯、草酸单酯和草酸酯类化合物在自由基脱羟基化反应中的共性和个性, 展望了自由基脱羟基化反应的未来和趋势.
陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623.
Jianqiang Chen, Gangguo Zhu, Jie Wu. Recent Advances in Radical-Based Dehydroxylation of Hydroxyl Groups via Oxalates[J]. Acta Chimica Sinica, 2023, 81(11): 1609-1623.

| [1] |
(a) Ertl P.; Schuhmann T. J. Nat. Prod. 2019, 82, 1258.
doi: 10.1021/acs.jnatprod.8b01022 |
|
(b) Henkel T.; Brunne R. M.; Müller H.; Reichel F. Angew. Chem. Int. Ed. 1999, 38, 643.
doi: 10.1002/(ISSN)1521-3773 |
|
| [2] |
For reviews, see: (a) Hatwig W. Tetrahedron 1983, 39, 2609.
doi: 10.1016/S0040-4020(01)91972-6 |
|
(b) Crich D.; Quintero L. Chem. Rev. 1989, 89, 1413.
doi: 10.1021/cr00097a001 |
|
| [3] |
(a) Zhang Z.; Gong L.; Zhou X.-Y.; Yan S.-S.; Li J.; Yu D.-G. Acta Chim. Sinica 2019, 77, 783.. (in Chinese)
doi: 10.6023/A19060208 |
|
( 张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚, 化学学报, 2019, 77, 783.)
doi: 10.6023/A19060208 |
|
|
(b) Chen J.-Q.; Tu X.; Tang Q.; Li K.; Xu L.; Wang S.; Ji M.; Li Z.; Wu J. Nat. Commun. 2021, 12, 5328.
doi: 10.1038/s41467-021-25628-x |
|
|
(c) Ji M.; Xu L.; Luo X.; Jiang M.; Wang S.; Chen J.; Wu J. Org. Chem. Front. 2021, 8, 6704.
doi: 10.1039/D1QO01368H |
|
|
(d) Yang M.; Ye B.; Chen J.; Wu J. Acta Chim. Sinica 2022, 80, 11.. (in Chinese)
doi: 10.6023/A21100457 |
|
|
( 杨民, 叶柏柏, 陈健强, 吴劼, 化学学报, 2022, 80, 11.)
doi: 10.6023/A21100457 |
|
|
(e) Chen J.-Q.; Tu X.; Qin B.; Huang S.; Zhang J.; Wu J. Org. Lett. 2022, 24, 642.
doi: 10.1021/acs.orglett.1c04082 |
|
|
(f) Chen J.-Q.; Chen Q.; Chen B.; Wu J. Org. Chem. Front. 2023, 10, 2018.
doi: 10.1039/D3QO00213F |
|
|
(g) Hou H.; Cheng Y.; Chen B.; Tung C.; Wu L. Chin. J. Org. Chem. 2023, 43, 1012.. (in Chinese)
doi: 10.6023/cjoc202211048 |
|
|
( 侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠, 有机化学, 2023, 43, 1012.)
doi: 10.6023/cjoc202211048 |
|
| [4] |
(a) Zheng X.; Dai X.-J.; Yuan H.-Q.; Ye C.-X.; Ma J.; Huang P.-Q. Angew. Chem. Int. Ed. 2013, 52, 3494.
doi: 10.1002/anie.201210088 pmid: 23420540 |
|
(b) Xie H.; Guo J.; Wang Y.-Q.; Wang K.; Guo P.; Su P.-F.; Wang X.; Shu X.-Z. J. Am. Chem. Soc. 2020, 142, 16787.
doi: 10.1021/jacs.0c07492 pmid: 23420540 |
|
|
(c) Pang X.; Su P.-F.; Shu X.-Z. Acc. Chem. Res. 2022, 55, 2491.
doi: 10.1021/acs.accounts.2c00381 pmid: 23420540 |
|
| [5] |
(a) Nguyen J. D.; Matsuura B. S.; Stephenson C. R. J. J. Am. Chem. Soc. 2014, 136, 1218.
doi: 10.1021/ja4113462 pmid: 29400958 |
|
(b) Nacsa E. D.; MacMillan D. W. C. J. Am. Chem. Soc. 2018, 140, 3322.
doi: 10.1021/jacs.7b12768 pmid: 29400958 |
|
|
(c) Zhao G.; Yao W.; Mauro J. N.; Ngai M.-Y. J. Am. Chem. Soc. 2021, 143, 1728.
doi: 10.1021/jacs.0c11209 pmid: 29400958 |
|
| [6] |
(a) Stache E. E.; Ertel A. B.; Rovis T.; Doyle A. G. ACS Catal. 2018, 8, 11134.
doi: 10.1021/acscatal.8b03592 pmid: 31367474 |
|
(b) Hu X.-Q.; Hou Y.-X.; Liu Z.-K.; Gao Y. Org. Chem. Front. 2020, 7, 2319.
doi: 10.1039/D0QO00643B pmid: 31367474 |
|
|
(c) Shao X.; Zheng Y.; Ramadoss V.; Tian L.; Wang Y. Org. Biomol. Chem. 2020, 18, 5994.
doi: 10.1039/D0OB01083A pmid: 31367474 |
|
|
(d) Guo H.-M.; Wu X. Nat. Commun. 2021, 12, 5365.
doi: 10.1038/s41467-021-25702-4 pmid: 31367474 |
|
| [7] |
(a) Dong Z.; MacMillan D. W. C. Nature 2021, 598, 451.
doi: 10.1038/s41586-021-03920-6 pmid: 35786873 |
|
(b) Sakai H. A.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 6185.
doi: 10.1021/jacs.2c02062 pmid: 35786873 |
|
|
(c) Intermaggio N. E.; Millet A.; Davis D. L.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 11961.
doi: 10.1021/jacs.2c04807 pmid: 35786873 |
|
| [8] |
(a) Barton D. H. R.; Crich D. Tetrahedron Lett. 1985, 26, 757.
doi: 10.1016/S0040-4039(00)89129-7 |
|
(b) Barton D. H. R.; Crich D.; Kretzschmar G. J. Chem. Soc., Perkin Trans. 1 1986, 39.
|
|
| [9] |
Lackner G. L.; Quasdorf K. W.; Overman L. E. J. Am. Chem. Soc. 2013, 135, 15342.
doi: 10.1021/ja408971t |
| [10] |
Lackner G. L.; Quasdorf K. W.; Pratsch G.; Overman L. E. J. Org. Chem. 2015, 80, 6012.
doi: 10.1021/acs.joc.5b00794 |
| [11] |
Gao C.; Li J.; Yu J.; Yang H.; Fu H. Chem. Commun. 2016, 52, 7292.
doi: 10.1039/C6CC01632D |
| [12] |
Chen X.; Luo X.; Peng X.; Guo J.; Zai J.; Wang P. Chem. Eur. J. 2020, 26, 3226.
doi: 10.1002/chem.v26.15 |
| [13] |
(a) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2015, 137, 11270.
doi: 10.1021/jacs.5b07678 |
|
(b) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2016, 138, 1724.
doi: 10.1021/jacs.6b00456 |
|
| [14] |
Lowry M. S.; Goldsmith J. L.; Slinker J. D.; Rohl R.; Pascal R. A.; Malliaras G. G.; Bernhard S. Chem. Mater. 2005, 17, 5712.
doi: 10.1021/cm051312+ |
| [15] |
Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Am. Chem. Soc. 2017, 139, 7192.
doi: 10.1021/jacs.7b04265 pmid: 28514145 |
| [16] |
Garnsey M. R.; Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Org. Chem. 2018, 83, 6958.
doi: 10.1021/acs.joc.7b02458 |
| [17] |
Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. Angew. Chem. Int. Ed. 2020, 59, 6268.
doi: 10.1002/anie.201916753 pmid: 31965671 |
| [18] |
Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. J. Org. Chem. 2020, 85, 15532.
doi: 10.1021/acs.joc.0c02273 pmid: 33197184 |
| [19] |
Abbas S. Y.; Zhao P.; Overman L. E. Org. Lett. 2018, 20, 868.
doi: 10.1021/acs.orglett.7b04034 |
| [20] |
Pitre S. P.; Muuronen M.; Fishman D. A.; Overman L. E. ACS Catal. 2019, 9, 3413.
doi: 10.1021/acscatal.9b00405 |
| [21] |
Dong J.; Wang Z.; Wang X.; Song H.; Liu Y.; Wang Q. J. Org. Chem. 2019, 84, 7532.
doi: 10.1021/acs.joc.9b00972 |
| [22] |
Lipp B.; Nauth A. M.; Opatz T. J. Org. Chem. 2016, 81, 6875.
doi: 10.1021/acs.joc.6b01215 |
| [23] |
Iwai K.; Takemura F.; Furue M.; Nozakura S.-I. Bull. Chem. Soc. Jpn. 1984, 57, 763.
doi: 10.1246/bcsj.57.763 |
| [24] |
Zuo Z.; MacMillan D. W. C. J. Am. Chem. Soc. 2014, 136, 5257.
doi: 10.1021/ja501621q |
| [25] |
Amos S. G. E.; Cavalli D.; Vaillant F. L.; Waser J. Angew. Chem. Int. Ed. 2021, 60, 23827.
doi: 10.1002/anie.v60.44 |
| [26] |
Li M.; Liu T.; Li J.; He H.; Dai H.; Xie J. J. Org. Chem. 2021, 86, 12386.
doi: 10.1021/acs.joc.1c01356 |
| [27] |
Luo J.; Zhang J. ACS Catal. 2016, 6, 873.
doi: 10.1021/acscatal.5b02204 |
| [28] |
Zhang X.; MacMillan D. W. C. J. Am. Chem. Soc. 2016, 138, 13862.
doi: 10.1021/jacs.6b09533 |
| [29] |
Guo L.; Song F.; Zhu S.; Li H.; Chu L. Nat. Commun. 2018, 9, 4543.
doi: 10.1038/s41467-018-06904-9 |
| [30] |
(a) Wang X.; Chen Y.; Liang P.; Chen J.-Q.; Wu J. Org. Chem. Front. 2022, 9, 4328.
doi: 10.1039/D2QO00741J |
|
(b) Chen M.; Sun W.; Yang J.; Yuan L.; Chen J.; Wu J. Green Chem. 2023, 25, 3857.
doi: 10.1039/D3GC01059G |
|
| [31] |
Guo L.; Tu H.-Y.; Zhu S.; Chu L. Org. Lett. 2019, 21, 4771.
doi: 10.1021/acs.orglett.9b01658 |
| [32] |
Li H.; Guo L.; Feng X.; Huo L.; Zhu S.; Chu L. Chem. Sci. 2020, 11, 4904.
doi: 10.1039/D0SC01471K |
| [33] |
Su J. Y.; Grünenfelder D. C.; Takeuchi K.; Reisman S. E. Org. Lett. 2018, 20, 4912.
doi: 10.1021/acs.orglett.8b02045 |
| [34] |
Brioche J. Tetrahedron Lett. 2018, 59, 4387.
doi: 10.1016/j.tetlet.2018.10.063 |
| [35] |
Gonzalez-Esguevillas M.; Miró J.; Jeffrey J. L.; MacMillan D. W. C. Tetrehedron 2019, 75, 4222.
doi: 10.1016/j.tet.2019.05.043 |
| [36] |
Troyano J. A.; Ballaschk F.; Jaschinski M.; Özkaya Y.; Gómez-Suárez A. Chem. Eur. J. 2019, 25, 14054.
doi: 10.1002/chem.v25.62 |
| [37] |
Vincent É.; Brioche J. Eur. J. Org. Chem. 2021, 2421.
|
| [38] |
Wang Q.; Yue L.; Bao Y.; Wang Y.; Kang D.; Gao Y.; Yuan Z. J. Org. Chem. 2022, 87, 8237.
doi: 10.1021/acs.joc.2c00664 |
| [39] |
Yan X. B.; Li C.-L.; Jin W.-J.; Guo P.; Shu X.-Z. Chem. Sci. 2018, 9, 4529.
doi: 10.1039/C8SC00609A |
| [40] |
Ye Y.; Chen H.; Sessler J. L.; Gong H. J. Am. Chem. Soc. 2019, 141, 820.
doi: 10.1021/jacs.8b12801 |
| [41] |
Gao M.; Sun D.; Gong H. Org. Lett. 2019, 21, 1645.
doi: 10.1021/acs.orglett.9b00174 |
| [42] |
Ye Y.; Chen H.; Yao K.; Gong H. Org. Lett. 2020, 22, 2070.
doi: 10.1021/acs.orglett.0c00561 |
| [43] |
Chen H.; Ye Y.; Tong W.; Fang J.; Gong H. Chem. Commun. 2020, 56, 454.
doi: 10.1039/C9CC07072A |
| [44] |
Ye Y.; Ma G.; Yao K.; Gong H. Synlett 2021, 32, 1625.
doi: 10.1055/a-1328-0352 |
| [45] |
Friese F. W.; Studer A. Angew. Chem. Int. Ed. 2019, 58, 9561.
doi: 10.1002/anie.v58.28 |
| [46] |
Ma G.; Chen C.; Talukdar S.; Zhao X.; Lei C.; Gong H. Chem. Commun. 2020, 56, 10219.
doi: 10.1039/D0CC04776G |
| [47] |
Guo P.; Wang K.; Jin W.-J.; Xie H.; Qi L.; Liu X.-Y.; Shu X.-Z. J. Am. Chem. Soc. 2021, 143, 513.
doi: 10.1021/jacs.0c12462 |
| [48] |
Chen Y.; Wang F.; Liu B.-X.; Rao W.-D.; Wang S.-Y. Org. Chem. Front. 2022, 9, 731.
doi: 10.1039/D1QO01614H |
| [49] |
Zhuo J.; Zhu C.; Wu J.; Li Z.; Li C. J. Am. Chem. Soc. 2022, 144, 99.
doi: 10.1021/jacs.1c11623 |
| [1] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [2] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [3] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [4] | 李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌. 氟烷基亚磺酸钠盐电化学合成α-氟烷基酮[J]. 化学学报, 2024, 82(2): 110-114. |
| [5] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [6] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| [7] | 崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明. CO2加氢制醇类催化剂的设计制备及性能研究进展[J]. 化学学报, 2023, 81(8): 1081-1100. |
| [8] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [9] | 何明慧, 叶子秋, 林桂庆, 尹晟, 黄心翊, 周旭, 尹颖, 桂波, 汪成. 卟啉基共价有机框架的光催化研究进展★[J]. 化学学报, 2023, 81(7): 784-792. |
| [10] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [11] | 任妍妍, 李欣, 韩英锋. 基于氮杂环卡宾蓝光有机自由基的合成及其光学性质研究★[J]. 化学学报, 2023, 81(7): 735-740. |
| [12] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [13] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [14] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [15] | 齐学平, 王飞, 张健. 后合成法构筑钛基金属有机框架及其应用[J]. 化学学报, 2023, 81(5): 548-558. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||