

收稿日期: 2023-10-10
网络出版日期: 2024-01-05
基金资助
中国科学院青年创新促进会(2020259); 国家自然科学基金(22271304); 上海市启明星计划(21QA1411200); 上海市自然科学基金(23ZR1476300)
The Regulation of Stereoselectivity in Radical Polymerization★
Received date: 2023-10-10
Online published: 2024-01-05
Supported by
Youth Innovation Promotion Association CAS(2020259); National Natural Science Foundation of China(22271304); Shanghai Rising-Star Program(21QA1411200); Shanghai Natural Science Foundation(23ZR1476300)
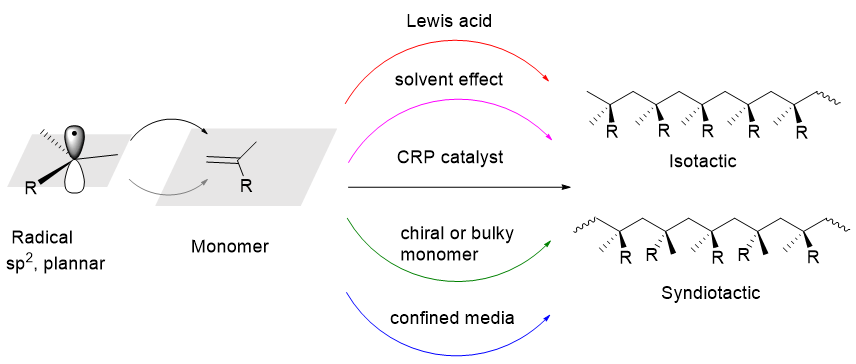
聚合物的物理和化学性能取决于其结构. 从组成聚合物的重复单元结构及比例到更为精细、复杂的聚合物微结构, 如聚合物的分子量及其分布、单体的序列排布(嵌段、梯度、接枝、交替等)、拓扑结构(星型、梳状、网状、刷状等)、末端官能团、立构规整度等都可能显著影响聚合物的性能, 人们对此进行了广泛而深入的研究. 其中, 聚合物的立构规整度的调控具有重要意义, 如丙烯的立体定向性配位聚合显示了巨大的商业价值. 虽然自由基聚合是高分子材料生产中应用最广泛的聚合技术之一, 但其难以对大分子结构进行控制. 可逆失活自由基聚合(RDRP)的发展使分子量的控制得到了显著改善. 然而, 对于所有形式的自由基聚合来说, 立体选择性的调控都极具挑战性. 这主要是由于自由基的高活性以及近平面特性, 使得单体加成时难以控制面的选择性, 生成的聚合物多为无规立构. 本综述从调控策略的角度对自由基聚合的立体选择性调控的相关研究进行了总结、评价和展望, 包括在限定环境中聚合、使用含手性辅基或大位阻取代基的单体、溶剂(氢键)效应、外加路易斯酸以及催化剂(或配体)效应等五个方面. 尽管这些调控手段已经获得了立体选择性自由基聚合的初步进展, 但总体而言仍然存在着单体范围受限、反应体系复杂、溶剂成本高、路易斯酸添加量大、催化效率低以及调控作用不明显等问题. 利用可控自由基聚合催化剂来调控立体选择性是极具潜力的, 未来可借鉴小分子的自由基不对称催化研究的经验来精心设计催化剂的结构并优化反应条件, 以此来拉近催化中心和聚合物链末端自由基的距离, 营造一种限域空间环境, 增强催化剂的结构对自由基加成聚合过程中的立体化学影响.
李雅宁 , 王晓艳 , 唐勇 . 自由基聚合的立体选择性调控★[J]. 化学学报, 2024 , 82(2) : 213 -225 . DOI: 10.6023/A23100445
The physical and chemical properties of polymer strongly depend on the structures of the polymer chain. The structure studies range from the structure of repeat units and their mole fractions to more detailed microstructures, such as molecular weight and its distribution, the sequence of monomer units (block, gradient, alternating, graft, etc.), topology (stars, combs, networks, brushes, etc.), the end-functionalities and tacticity. Among them, the regulation of the polymer tacticity is greatly significant. For example, the stereospecific coordination polymerization of propylene has shown great commercial value. The control of macromolecular structure is difficult for free radical polymerization, although it is one of the most widely used polymerization techniques in the production of polymer materials. The development of reversible deactivated radical polymerization (RDRP) has significantly improved the control over the molecular weight of polymer. However, the regulation of stereoselectivity is extremely challenging for all forms of radical polymerization. One of the reasons is because a terminal carbon of propagating radical takes essentially a neutral sp2 planar-like structure without counter species in contrast to an anionic sp3 pyramidal or cationic sp2 planar-like structure with counter ion or chiral catalyst site. This brings about a non-stereospecific radical propagation, and results in the energy difference between two enantiomers of an active radical species is small and the energy barrier between the enantiomers is low compared to the thermal energy at the polymerization temperature. In this review, the regulation of stereoselectivity in free radical polymerization are comprehensively summarized, evaluated, and prospected from the perspective of regulation strategies, including polymerization in restricted environment, using monomer containing chiral or bulky substituents, solvent (hydrogen bond) effect, the addition of Lewis acid and catalyst or ligand effect. Although these strategies have achieved some preliminary progress, there are still some problems in general, such as limited monomer scope, high solvent cost, complex reaction system, high Lewis acid loading, low catalytic efficiency and insignificant regulatory effect. The use of controlled radical polymerization catalysts to regulate stereoselectivity is of great potential. In the future, the studies on the radical asymmetric catalysis of small molecule can be used for reference to carefully design the structure of the catalyst and optimize the reaction conditions. In this way, the distance between the catalytic center and the terminal radical of the polymer chain is narrowed, and a confined space environment is created to enhance the stereochemical influence of the catalyst structure on the radical addition polymerization process.

Key words: radical polymerization; stereoselectivity; mechanism; tactic polymer; catalyst
| [1] | (a) Matyaszewski, K.; Davis, T. P. Handbook of Radical Polymerization, Wiley-Interscience, Hoboken, 2002. |
| [1] | (b) Kamigaito, M.; Ando, T.; Sawamoto, M. Chem. Rev. 2001, 101, 3689. |
| [1] | (c) Matyjaszew- ski, K.; Xia, J. Chem. Rev. 2001, 101, 2921. |
| [1] | (d) Li, H.-M.; Wang, J.; Ni, Y.-Z.; Zhou, Y.-F.; Yan, D.-Y. Acta Chim. Sinica 2016, 74, 415. (in Chinese) |
| [1] | (李惠梅, 王洁, 倪云洲, 周永丰, 颜德岳, 化学学报, 2016, 74, 415.) |
| [1] | (e) Li, L.-L.; Xiang, Y.-Y.; Liu, H.; Ma, S.-H.; Li, B.; Ma, Z.-Q.; Wei, Q.-B.; Yu, B.; Zhou, F. Acta Chim. Sinica 2021, 79, 353. (in Chinese) |
| [1] | (李乐乐, 向阳阳, 刘欢, 麻拴红, 李斌, 马正峰, 魏强兵, 于波, 周峰, 化学学报, 2021, 79, 353.) |
| [1] | (f) Lin, D.-N.; Zhang, L.; Tan, J.-B. Acta Polym. Sinica 2023, 54, 761. (in Chinese) |
| [1] | (林冬妮, 张力, 谭剑波, 高分子学报, 2023, 54, 761.) |
| [2] | (a) Teator, A. J.; Varner, T. P.; Knutson, P. C.; Sorensen, C. C.; Leibfarth, F. A. ACS Macro Lett. 2020, 9, 1638. |
| [2] | (b) Li, Y.; Wang, X.-Y.; Tang, Y. Acta Chim. Sinica 2021, 79, 1320. (in Chinese) |
| [2] | (李勇, 王晓艳, 唐勇, 化学学报, 2021, 79, 1320.) |
| [2] | (c) Jiang, Y.; Zhang, Z.; Li, S.; Cui, D. Angew. Chem., Int. Ed. 2022, 61, e202112966. |
| [2] | (d) Mariott, W. R.; Chen, E. Y.-X. J. Am. Chem. Soc 2002, 124, 5612. |
| [2] | (e) Zhang, X.; Yang, Z.; Jiang, Y.; Liao, S. J. Am. Chem. Soc 2021, 144, 679. |
| [2] | (f) Zhu, H.; Jiang, Y.; Yang, Z.; Zhang, X.; Liao, S. Giant 2023, 14, 100151. |
| [3] | (a) Sciannamea, V.; Jerome, R.; Detrembleur, C. Chem. Rev. 2008, 108, 1104. |
| [3] | (b) Fairbanks, B. D.; Gunatillake, P. A.; Meagher, L. Adv. Drug Delivery Rev. 2015, 91, 141. |
| [4] | Kamigaito, M.; Satoh, K. Macromolecules 2008, 41, 269. |
| [5] | Satoh, K.; Kamigaito, M. Chem. Rev. 2009, 109, 5120. |
| [6] | Wegner, G. Z. Naturforsch., B 1969, 24, 824. |
| [7] | Matsumoto, A.; Matumura, T.; Aoki, S. J. Chem. Soc., Chem. Commun. 1994, 1389. |
| [8] | Hema, K.; Ravi, A.; Raju, C.; Pathan, J. R.; Rai, R.; Sureshan, K. M. Chem. Soc. Rev. 2021, 50, 4062. |
| [9] | Grisenthwaite, R. J.; Hunter, R. F. Chem. Ind. 1959, 433. |
| [10] | Minanawa, M.; Yamada, H.; Yamaguchi, K.; Yoshii, F. Macromolecules 1992, 25, 503. |
| [11] | Farina, M.; Audisio, G.; Natta, G. J. Am. Chem. Soc. 1967, 89, 5071. |
| [12] | Bartels, T.; Tan, Y. Y.; Challa, G. J. Polym. Sci., Polym. Chem. Ed. 1977, 15, 341 |
| [13] | (a) Narumi, A.; Baba, H.; Akabane, T.; Saito, Y.; Ohno, S.; Togashi, D.; Enomoto, K.; Kikuchi, M.; Haba, O.; Kawaguchi, S. Macromolecules 2015, 48, 3395. |
| [13] | (b) Hwang, J. H.; Lee, H. C.; Antonietti, M.; Schmidt, B. V. K. J. Polym. Chem. 2017, 8, 6204. |
| [14] | Nakano, T.; Okamoto, Y. Chem. Rev. 2001, 101, 4013. |
| [15] | Porter, N. A.; Allen, T. R.; Breyer, R. A. J. Am. Chem. Soc. 1992, 114, 7676. |
| [16] | (a) Wu, W. X.; Mcphail, A. T.; Porter, N. A. J. Org. Chem. 1994, 59, 1302. |
| [16] | (b) Tanaka, H.; Niwa, M. Polymer 2008, 49, 3693. |
| [16] | (c) Nakano, H.; Kinjo, N.; Hidaka, Y.; Okamoto, Y. Polym. J. 1999, 31, 464. |
| [16] | (d) Nakano, T.; Sogah, D. Y. J. Am. Chem. Soc. 1995, 117, 534. |
| [16] | (e) Fujita, T.; Yamago, S. Chem. Eur. J. 2015, 21, 18547. |
| [17] | Tanaka, H.; Maki, K.; Matsubara, Y. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 184. |
| [18] | (a) Lando, J. B.; Litt, M.; Kumar, N. G.; Shimko, T. M. J. Polym. Sci., Part C: Symp. 1974, 44, 203. |
| [18] | (b) Yamada, K; Nakano, T.; Okamoto, Y. Macromolecules 1998, 31, 7598. |
| [18] | (c) Isobe, Y.; Yamada, K.; Nakano, T.; Okamoto, Y. J. Polym, Sci., Part A: Polym. Chem. 2000, 38, 4693. |
| [18] | (d) Zhang, J.; Liu, W.; Nakamo, T.; Okamoto, Y. Polym. J. 2000, 32, 694. |
| [18] | (e) De?irmenci, I.; Eren, ?.; Aviyente, V.; De Sterck, B.; Hemelsoet, K.; Van Speybroeck, V.; Waroquier, M. Macromolecules 2010, 43, 5602. |
| [18] | (f) Miura, Y.; Satoh, A.; Nishizawa, Y.; Okamoto, Y.; Kakuchi, T. Macromolecules 2005, 38, 1041. |
| [18] | (g) Shim, S.-H.; Ham, M.-K.; Huh, J.; Kwon, Y.-K.; Kwark, Y.-J. Polym. Chem. 2013, 4, 5449. |
| [18] | (h) Li, N.; Ding, D.; Pan, X.; Zhang, Z.; Zhu, J.; Boyer, C.; Zhu, X. Polym. Chem. 2017, 8, 6024. |
| [18] | (i) Wan, D.; Satoh, K.; Kamigaito, M. Macromolecules 2006, 39, 6882. |
| [18] | (j) Tao, Y.; Satoh, K.; Kamigaito, M. Macromol. Rapid Commun. 2010, 32, 226. |
| [19] | Matsumoto, A.; Nakamura, S. J. Appl. Polym. Sci. 1999, 74, 290. |
| [20] | Habaue, S.; Baraki, H.; Okamoto, Y. Polym. J. 2000, 32, 1017. |
| [21] | Isobe, Y.; Nakano, T.; Okamoto, Y. J. Polym. Sci., Part A: Polym. Chem. 2001, 39, 1463. |
| [22] | Noble, B. N.; Smith, L. M.; Coote, M. L. Polym. Chem. 2014, 5, 4974. |
| [23] | Schaubach, S.; Wang, X.-Y.; Li, J.-F.; Sun, X.-L.; Wang, S. R.; Tang, Y. Polym. Chem. 2018, 9, 4711. |
| [24] | Samal, S.; Thompson, B. C. ACS Macro Lett. 2018, 7, 1161. |
| [25] | Isobe, Y.; Fujioka, D.; Habaue, S.; Okamoto, Y. J. Am. Chem. Soc. 2001, 123, 7180. |
| [26] | Lutz, J. F.; Neugebauer, D.; Matyjaszewski, K. J. Am. Chem. Soc. 2003, 125, 6986. |
| [27] | Shanmugam, S.; Boyer, C. J. Am. Chem. Soc. 2015, 137, 9988. |
| [28] | Sun, Y.; Fu, L. Y.; Olszewski, M.; Matyjaszewski, K. Macromol. Rapid Commun. 2019, 40, 1800877. |
| [29] | (a) Imamura, Y.; Fujita, T.; Kobayashi, Y.; Yamago, S. Polym Chem. 2020, 11, 7042. |
| [29] | (b) Park, B.; Imamura, Y.; Yamago, S. Polym. J. 2021, 53, 515. |
| [30] | (a) Jiang, X.; Xiong, W.; Deng, S.; Lu, F.-D.; Jia, Y.; Yang, Q.; Xue, L.-Y.; Qi, X.; Tunge, J. A.; Lu, L.-Q.; Xiao, W.-J. Nat. Catal. 2022, 5, 788. |
| [30] | (b) Liu, X.; Liu, B.; Liu, Q. Angew. Chem., Int. Ed. 2020, 59, 6750. |
| [30] | (c) Li, L.-J.; He, Y.; Yang, Y.; Guo, J.; Lu, Z.; Wang, C.; Zhu, S.; Zhu, S.-F. CCS Chem. 2023, DOI: 10.31635/ccschem.023.202303412. |
| [30] | (d) Wu, Z. Q.; Peng, C. H.; Fu, X. F. Polym. Chem. 2020, 11, 4387. |
| [31] | Zhang, X.; Lin, F.; Cao, M.; Zhong, M. Nat. Synth. 2023, https://doi.org/10.1038/s44160-023-00311-9. |
| [32] | Haddleton, D. M.; Duncalf, D. J.; Kululj, D.; Heming, A. M.; Shooter, A. J.; Clark, A. J. J. Mater. Chem. 1998, 8, 1525. |
| [33] | Yu, B.; Ruckenstein, E. J. Polym. Sci., Part A: Polym. Chem. 1999, 37, 4191. |
| [34] | Johnson, R. M.; Ng, C.; Samson, C. C. M.; Fraser, C. L. Macromolecules 2000, 33, 8618. |
| [35] | Stoffelbach, F.; Richard, P.; Poli, R.; Jenny, T.; Savary, C. Inorg. Chim. Acta 2006, 359, 4447. |
| [36] | Kameyama, M.; Kamigata, N.; Kobayashi, M. J. Org. Chem. 1987, 52, 3312. |
| [37] | Iizuka, Y.; Li, Z.; Satoh, K.; Kamigaito, M.; Okamoto, Y.; Ito, J.; Nishiyama, H. Eur. J. Org. Chem. 2007, 782. |
| [38] | (a) Zhou, J.; Tang, Y. Chem. Soc. Rev. 2005, 34, 664. |
| [38] | (b) Liao, S.-H.; Sun, X.-L.; Tang, Y. Acc. Chem. Res. 2014, 47, 2260. |
| [38] | (c) Sun, X.-L; Tang, Y. Acta Polym. Sinica 2017, 7, 1019. (in Chinese) |
| [38] | (孙秀丽, 唐勇, 高分子学报, 2017, 7, 1019.) |
| [39] | (a) Wang, X.-Y.; Sun, X.-L.; Wang, F.; Tang, Y. ACS Catal. 2017, 7, 4692. |
| [39] | (b) Chen, Z.-H.; Wang, X.-Y.; Sun, X.-L.; Li, J.-F.; Zhu, B.-H.; Tang, Y. Macromolecules 2019, 52, 9792. |
| [39] | (c) Chen, Z.-H.; Ma, Y.; Wang, X.-Y.; Sun, X.-L.; Li, J.-F.; Zhu, B.-H.; Tang, Y. ACS Catal. 2020, 10, 14127. |
| [39] | (d) Wang, X.-Y.; Sun, X.-L.; Chen, Z.-H.; Wang, F.; Wang, R. S.; Tang, Y. Polym. Chem. 2018, 9, 4309. |
| [39] | (e) Wang, X.-Y.; Chen, Z.-H.; Sun, X.-L.; Tang, Y. Polymer 2019, 178, 121630. |
| [39] | (f) Ma, Y.; Yang, H.-M.; Chen, Z.-H.; Li, Y.-N.; Li, J.-F.; Sun, X.-L.; Wang, X.-Y.; Tang, Y. Polym. Chem. 2021, 12, 6606. |
/
| 〈 |
|
〉 |