

苯并噁唑取代氨基酚氧基锌氯化物催化外消旋丙交酯开环聚合研究
Ring-opening Polymerization of rac-Lactide Catalyzed by Zinc Chloride Complexes Supported by Aminophenolate Ligands Bearing a Benzoxazolyl Group
Received date: 2024-12-23
Online published: 2025-01-07
Supported by
National Natural Science Foundation of China(21871082)
合成了4个苯并噁唑取代的氨基酚氧基锌氯化物1~4, 通过1H NMR、13C NMR和元素分析对其结构进行表征. 对配合物1通过X-ray单晶衍射分析确定了其晶体结构. 该系列锌氯化物稳定性较高, 可以催化工业级外消旋丙交酯(rac-LA)开环聚合, 并具有较好的催化活性, 主要得到环状聚合物和部分线性聚合物; 在添加苄醇的条件下, 得到苄氧基和羟基封端的线性聚合物, 同时有较多环状聚合物生成. 骨架氮原子上取代基对配合物催化性能有较大影响, 具直链烷基取代的配合物1催化活性最高, 在80 ℃下, 以环氧环己烷为溶剂, 催化50000倍物质的量工业级rac-LA开环聚合, 转化频率(TOF)值达到2716 h-1, 得到分子量为Mn=2.03×104 g/mol的聚合物. 骨架氮上环己基取代的配合物3对rac-LA聚合的等规选择性最高, 在80 ℃聚合时达到Pm=0.67; 降低聚合温度至室温, 等规选择性提高至Pm=0.70. 通过改变单体浓度, 利用配合物1实现了分子量最高达到Mn=3.10×104 g/mol的环状聚丙交酯的合成. 基于低聚物的基质辅助激光解吸电离飞行时间(MALDI-TOF)质谱分析, 认为该系列配合物通过先引发环氧环己烷开环得到金属烷氧基物种, 进而通过配位插入机理催化外消旋丙交酯开环聚合, 并通过端对端环化以及非选择性环化得到环状聚丙交酯.
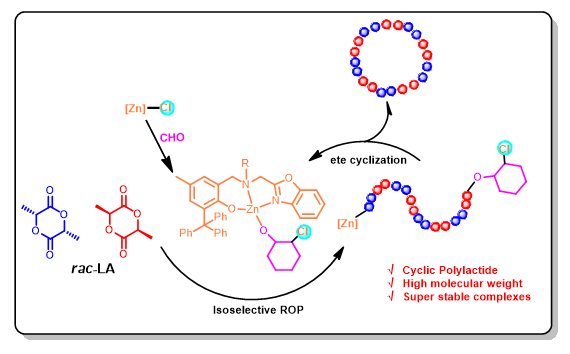
关键词: 苯并噁唑取代氨基酚配体; 锌氯化物; 外消旋丙交酯; 开环聚合; 环状聚丙交酯
王玉娜 , 王超 , 马海燕 . 苯并噁唑取代氨基酚氧基锌氯化物催化外消旋丙交酯开环聚合研究[J]. 化学学报, 2025 , 83(1) : 25 -35 . DOI: 10.6023/A24090275
Four zinc chloride complexes supported by aminophenolate ligands bearing a benzoxazolyl group were synthesized via the reactions of the corresponding sodium salts of the proligands HL1-4 with one equiv. of zinc dichloride at room temperature respectively, and their structures were well-characterized by 1H NMR, 13C NMR and elemental analysis (EA) methods. The molecular structure of complex 1 was further determined by X-ray single crystal diffraction analysis, where the phenoxy oxygen atom, the skeleton N atom, and the benzoxazolyl N atom of the ligand all coordinate with the metal center, forming a disordered tetrahedral coordination geometry around the metal center together with the chloride ligand. This series of zinc chloride complexes showed high stability, and could be applied to catalyze the ring-opening polymerization (ROP) of industrial grade racemic lactide (rac-LA) with good activities. The substituent on the skeleton N atom exhibited a significant impact on the catalytic performance of the complex. Complex 1 with a linear n-butyl group on the skeleton N atom displayed the highest catalytic activity. At 80 ℃, 50000 equiv. of industrial grade rac-LA could be polymerized by complex 1 using cyclohexane oxide (CHO) as a solvent, and a high turnover frequency (TOF) value of 2716 h-1 was reached, resulting in a cyclic polymer with a molecular weight of Mn=2.03×104 g/mol. Complex 3 with a cyclohexyl group on the skeleton N atom showed the highest isotactic selectivity among these zinc chloride complexes toward the ROP of rac-LA, at 80 ℃, Pm=0.67, which could be increased to Pm=0.70 when the polymerization was carried out at 25 ℃. By adopting a high monomer concentration of 14 mol/L in the polymerization, cyclic polylactide with a molecular weight of Mn=3.10×104 g/mol was achieved using complex 1. Based on the matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectroscopic analysis of oligomers, it was found that in the absence of benzyl alcohol, the polymer formed was mainly cyclic, containing small amount of linear chains end-capped with -OH/-COOH, -OH/-OC6H10Cl groups etc.; while in the presence of benzyl alcohol, the resultant polymer was found to be a mixture of linear ones end-capped with -OH/-OBn and cyclic ones, both in a reasonable amount. It is thus suggested that, in the absence of external alcohol a coordination-insertion polymerization mechanism is involved, which integrates with end-to-end and non-selective cyclization to produce mainly cyclic polymers, wherein CHO acts as activator to zinc chloride complexes by forming zinc alkoxide species via the nucleophilic attack of chlorine to the coordinated CHO; when benzyl alcohol was used, it acts as a chain transfer reagent with the active propaga- tion chain end-capped with -OH/-OC6H10Cl to generate zinc benzyloxide species, which initiates the ROP of rac-LA to form linear polymers end-capped with -OH/-OBn groups, meanwhile the cyclization of the active propagation chain end-capped with -OH/-OC6H10Cl still takes place in this system.

| [1] | Zhu, Y.; Romain, C.; Williams, C. K. Nature 2016, 540, 354. |
| [2] | Keram, M.; Ma, H. Acta Chim. Sinica 2018, 76, 121 (in Chinese) |
| [2] | ( 布美热木?克里木, 马海燕, 化学学报, 2018, 76, 121.) |
| [3] | Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biomacromolecules 2019, 20, 1465. |
| [4] | Mehta, R.; Kumar, V.; Bhumia, H.; Upadhyay, S. N. J. Macromol. Sci. Part C: Polym. Rev. 2005, 45, 325. |
| [5] | Drumright, R. E.; Gruber, P. R.; Henton, D. E. Adv. Mater. 2000, 12, 1841. |
| [6] | Ungpittagul, T.; Wongmahasirikun, P.; Phomphrai, K. Dalton Trans. 2020, 49, 8460. |
| [7] | Haque, F. M.; Grayson, S. M. Nat. Chem. 2020, 12, 433. |
| [8] | Pangilinan, K.; Advincula, R. Polym. Int. 2014, 63, 803. |
| [9] | Chang, Y. A.; Waymouth, R. M. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 2892. |
| [10] | Laurent, B. A.; Grayson, S. M. Chem. Soc. Rev. 2009, 38, 2202. |
| [11] | Hu, C.; Louisy, E.; Fontaine, G.; Bonnet, F. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3175. |
| [12] | Piedra-Arroni, E.; Ladaviere, C.; Amgoune, A.; Bourissou, D. J. Am. Chem. Soc. 2013, 135, 13306. |
| [13] | Shaik, M.; Peterson, J.; Du, G. Macromolecules 2019, 52, 157. |
| [14] | Liu, J. Y.; Liu, S.; Suo, H. Y.; Qin, Y. S. Acta Polym. Sin. 2024, 55, 770 (in Chinese). |
| [14] | ( 刘娇玉, 刘爽, 索泓一, 秦玉升, 高分子学报, 2024, 55, 770.) |
| [15] | Culkin, D. A.; Jeong, W.; Csihony, S.; Gomez, E. D.; Balsara, N. P.; Hedrick, J. L.; Waymouth, R. M. Angew. Chem., Int. Ed. 2007, 46, 2627. |
| [16] | Shin, E. J.; Brown, H. A.; Gonzalez, S.; Jeong, W.; Hedrick, J. L.; Waymouth, R. M. Angew. Chem., Int. Ed. 2011, 50, 6388. |
| [17] | Stukenbroeker, T. S.; Solis-Ibarra, D.; Waymouth, R. M. Macromolecules 2014, 47, 8224. |
| [18] | Chang, Y. A.; Rudenko, A. E.; Waymouth, R. M. ACS Macro Lett. 2016, 5, 1162. |
| [19] | Dunn, A. L.; Landis, C. R. Macromolecules 2017, 50, 2267. |
| [20] | Weil, J.; Mathers, R. T.; Getzler, Y. D. Y. L. Macromolecules 2012, 45, 1118. |
| [21] | Castro-Osma, J. A.; Alonso-Moreno, C.; García-Martinez, J. C.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Lara-Sánchez, A.; Otero, A. Macromolecules 2013, 46, 6388. |
| [22] | Weidner, S. M.; Kricheldorf, H. R. Macromol. Chem. Phys. 2017, 218, 1600331. |
| [23] | Anker, M.; Balasanthiran, C.; Balasanthiran, V.; Chisholm, M. H.; Jayaraj, S.; Mathieu, K.; Piromjitpong, P.; Praban, S.; Raya, B.; Simonsick, W. J. Dalton Trans. 2017, 46, 5938. |
| [24] | Praban, S.; Piromjitpong, P.; Balasanthiran, V.; Jayaraj, S.; Chisholm, M. H.; Tantirungrotechai, J.; Phomphrai, K. Dalton Trans. 2019, 48, 3223. |
| [25] | Praban, S.; Yimthachote, S.; Kiriratnikom, J.; Chotchatchawankul, S.; Tantirungrotechai, J.; Phomphrai, K. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 2104. |
| [26] | Kricheldorf, H. R.; Weidner, S. M.; Meyer, A. Polym. Chem. 2020, 11, 2182. |
| [27] | Kerr, R. W. F.; Ewing, P. M. D. A.; Raman, S. K.; Smith, A. D.; Williams, C. K.; Arnold, P. L. ACS Catal. 2021, 11, 1563. |
| [28] | Piromjitpong, P.; Ratanapanee, P.; Thumrongpatanaraks, W.; Kongsaeree, P.; Phomphrai, K. Dalton. Trans. 2012, 41, 12704. |
| [29] | Chen, C.; Cui, Y.; Mao, X.; Pan, X.; Wu, J. Macromolecules 2017, 50, 83. |
| [30] | Si, G.; Zhang, S.; Pang, W.; Wang, F.; Tan, C. Polymer 2018, 154, 148. |
| [31] | Impemba, S.; Della Monica, F.; Grassi, A.; Capacchione, C.; Milione, S. ChemSusChem 2020, 13, 141. |
| [32] | Goonesinghe, C.; Jung, H.-J.; Roshandel, H.; Diaz, C.; Baalbaki, H. A.; Nyamayaro, K.; Ezhova, M.; Hosseini, K.; Mehrkhodavandi, P. ACS Catal. 2022, 12, 7677. |
| [33] | Hu, J.; Kan, C.; Ma, H. Inorg. Chem. 2018, 57, 11240. |
| [34] | Gong, Y.; Ma, H. Chem. Commun. 2019, 55, 10112. |
| [35] | Hu, J.; Kan, C.; Wang, H.; Ma, H. Macromolecules 2018, 51, 5304. |
| [36] | Wang, H.; Ma, H. Macromolecules 2024, 57, 6156. |
| [37] | Industrial grade lactides contain trace amounts of protonic impurities such as water and lactic acid. For the vast majority of metal complexes reported in literature, complete decomposition usually occurs when industrial grade lactides are adopted for polymerization directly without purfication. Although zinc chloride complexes show significantly increased tolerance to impurities, they still undergo decomposition to a certain degree, leading to a decrease in catalytic activity. |
| [38] | Larrow, J. F.; Jacobsen, E. N.; Gao, Y.; Hong, Y.; Nie, X.; Zepp, C. M. J. Org. Chem. 1994, 59, 1939. |
/
| 〈 |
|
〉 |