

“两步法”合成基于二硫键连接子的奥曲肽-阿霉素偶联物
收稿日期: 2025-02-02
网络出版日期: 2025-04-08
基金资助
国家自然科学基金(22177058); 山东省泰山学者青年专家项目(tsqn202312168); 山东省自然科学基金优青项目(ZR2024YQ061)
Two Step Synthesis of Octreotide-doxorubicin Conjugates Based on Disulfide Bond Linker
Received date: 2025-02-02
Online published: 2025-04-08
Supported by
National Natural Science Foundation of China(22177058); Taishan Scholar Project of Shandong Province(tsqn202312168); Natural Science Foundation of Shandong Province(ZR2024YQ061)
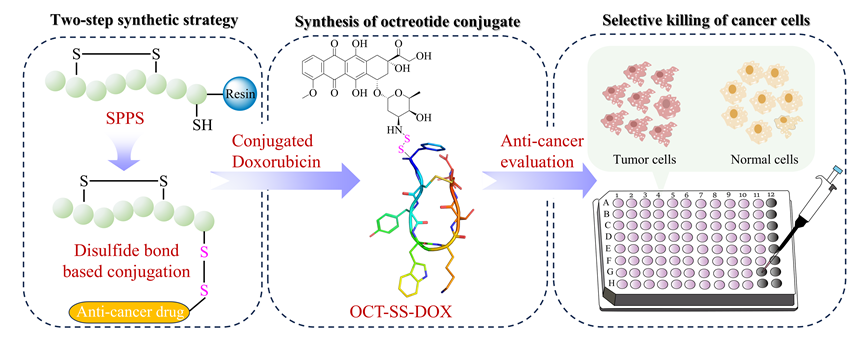
癌症仍然是一个全球性的健康挑战, 开发具有新骨架的新型抗癌药物具有重要意义. 多肽-药物偶联物(PDCs)是新型靶向抗癌药物, 具有对肿瘤组织穿透性强、生产成本低及易于结构改造等优势, 可显著提高细胞毒性药物的靶向性和抗肿瘤活性. 奥曲肽是一种可特异性靶向肿瘤细胞表面生长抑素受体2 (SSTR2)的环肽, 在PDCs药物研发中得到广泛应用. 二硫键是PDCs分子中常用的可裂解连接基团. 以往合成含有二硫键连接子的奥曲肽-小分子药物偶联物一般采用“三步法”策略, 但合成路线繁琐且产率较低. 基于此, 本研究开发了“两步法”合成策略, 即先在固相上合成含有分子内二硫键和巯基末端的奥曲肽衍生物, 再通过液相反应与含有巯基的小分子药物共价偶联. 为了实现含巯基末端奥曲肽的高效合成, 本研究探索了不同的固相缩合路线和切肽体系, 综合考虑色谱纯度和分离收率等, 筛选出最佳合成路线SPPS-2和最优切肽体系. 为了进一步验证SPPS-2合成路线的普适性, 探讨了不同树脂和切肽时间对合成效率的影响. 固相高效合成巯基奥曲肽(OCT-SH)后, 将化疗药物阿霉素(DOX)通过液相反应与OCT-SH共价偶联, 形成奥曲肽-阿霉素偶联物. 体外抗肿瘤实验表明, 奥曲肽-阿霉素偶联物具有较强的抗肿瘤活性, 可以显著降低阿霉素对正常细胞的毒性并实现对肿瘤细胞的高选择性. 细胞摄取实验表明, 阿霉素的共价偶联对奥曲肽的细胞摄取能力没有明显影响. 本研究利用“两步法”合成策略, 建立了最佳的固相缩合路线和切肽体系, 实现了基于二硫键连接子的奥曲肽-阿霉素偶联物的高效合成. 本研究为固相合成含有多对二硫键的PDCs分子提供了重要参考, 为开发新型抗肿瘤药物提供了启发.
于千尧 , 孟铭 , 姚景方 , 杜姗姗 , 齐昀坤 . “两步法”合成基于二硫键连接子的奥曲肽-阿霉素偶联物[J]. 化学学报, 2025 , 83(4) : 341 -353 . DOI: 10.6023/A25020034
Cancer is still a global health challenge and developing novel anticancer drugs with new skeletons is of great importance. Recently, peptide-based agents have become promising candidates for anticancer treatments, especially for the treatment of drug-resistant refractory malignancies. Peptide drug conjugates (PDCs) are novel targeted anticancer drugs with advantages including high permeability to cancer tissues, low production costs, and ease of structural optimization. PDCs could significantly enhance the selectivity and anticancer activity of cytotoxic drugs. Octreotide, which is a cyclic peptide inherently containing one pair of disulfide bonds, could specifically target the somatostatin receptor 2 (SSTR2) on the surface of cancer cells, leading to its widespread clinical applications. The reducible disulfide bonds serve as a commonly utilized cleavable linker in PDCs. However, traditional synthesis of octreotide-drug conjugates based on disulfide bond linkers generally used the “three-step” strategy, including complex synthetic procedure and inducing low isolation yields. To address this issue, we developed a “two-step” synthetic strategy. First, octreotide containing one disulfide bond and the free thiol group (OCT-SH) was synthesized by the straightforward solid-phase peptide synthesis (SPPS). Subsequently, the OCT-SH was conjugated to small molecule drugs through site-specific liquid-phase reaction. To achieve the efficient and economic synthesis of OCT-SH, various SPPS based strategies and peptide cleavage systems were explored. Considering the chromatographic purity and synthetic yield, the optimal synthetic route (SPPS-2) and peptide cleavage system were established. To further validate the robustness of SPPS-2, the influences of different solid resins and peptide cleavage time on synthetic efficiency were investigated. After the efficient manual synthesis of OCT-SH, the promising chemotherapeutic drug doxorubicin (DOX) was covalently coupled to OCT-SH through liquid-phase reaction, affording the octreotide-doxorubicin conjugate. In vitro anticancer studies indicated that the octreotide-doxorubicin conjugate exhibited potent anticancer activity, significantly reducing toxicity to normal cells while achieving selectivity towards cancer cells. The cellular uptake experiment indicated that the covalent conjugation of doxorubicin did not significantly impair the cellular uptake potency of octreotide by cancer cells. Collectively, this study established the “two-step” synthetic strategy, enabling efficient and straightforward preparation of octreotide-doxorubicin conjugates containing the disulfide bond linker. This work also provides valuable references for the SPPS based synthesis of PDCs containing multiple disulfide bonds and inspires the development of novel PDCs for cancer therapy.

| [1] | Siegel, R. L.; Giaquinto, A. N.; Jemal, A. CA-Cancer J. Clin. 2024, 74, 12. |
| [2] | Anderson, R. L.; Balasas, T.; Callaghan, J.; Coombes, R. C.; Evans, J.; Hall, J. A.; Kinrade, S.; Jones, D.; Jones, P. S.; Jones, R.; Marshall, J. F.; Panico, M. B.; Shaw, J. A.; Steeg, P. S.; Sullivan, M.; Tong, W.; Westwell, A. D.; Ritchie, J. W. A.; Berg, R.; Drysdale, M.; Eccles, S.; Elvin, P.; Harris, A.; Ireson, C.; Machesky, L.; McLeod, R.; Muschel, R.; Newell, H.; Pittman, M.; Roman, B.; Santos, C.; Sibson, N.; Smith, A.; Waddell, I. Nat. Rev. Clin. Oncol. 2019, 16, 185. |
| [3] | Ma, Q. X.; Xu, Q. Q.; Zhao, J. J.; Zhang, W. W.; Wang, Q.; Fang, J.; Lu, Z. M.; Liu, J.; Ma, L. N. Cancer Lett. 2021, 520, 243. |
| [4] | Bargh, J. D.; Isidro-Llobet, A.; Parker, J. S.; Spring, D. R. Chem. Soc. Rev. 2019, 48, 4361. |
| [5] | Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Signal Transduct. Target. Ther. 2022, 7, 93. |
| [6] | Paul, S.; Konig, M. F.; Pardoll, D. M.; Bettegowda, C.; Papadopoulos, N.; Wright, K. M.; Gabelli, S. B.; Ho, M.; van Elsas, A.; Zhou, S. Nat. Rev. Cancer 2024, 24, 399. |
| [7] | Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Acta Pharm. Sin. B 2023, 13, 498. |
| [8] | Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Int. J. Oncol. 2020, 57, 678. |
| [9] | Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D. G.; Zhang, W.-D. Nat. Prod. Rep. 2021, 38, 7. |
| [10] | Cooper, B. M.; Iegre, J.; O' Donovan, D. H.; Halvarsson, M. O.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 1480. |
| [11] | Dean, T. T.; Jelu-Reyes, J.; Allen, A. C.; Moore, T. W. J. Med. Chem. 2024, 67, 1641. |
| [12] | He, H.; Deng, X.; Wang, Z.; Chen, J. Eur. J. Med. Chem. 2025, 284, 117204. |
| [13] | Sagar, B.; Gupta, S.; Verma, S. K.; Reddy, Y. V. M.; Shukla, S. Eur. J. Med. Chem. 2025, 283, 117131. |
| [14] | Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; Liang, S.; Zeng, Y.; Liu, Z. J. Med. Chem. 2019, 62, 11108. |
| [15] | Vitale, I.; Yamazaki, T.; Wennerberg, E.; Sveinbjornsson, B.; Rekdal, O.; Demaria, S.; Galluzzi, L. Trends Cancer 2021, 7, 557. |
| [16] | Yin, H.; Chen, X.-T.; Chi, Q.-N.; Ma, Y.-N.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Acta Pharmacol. Sin. 2023, 44, 201. |
| [17] | Yin, H.; Fu, X.-Y.; Gao, H.-Y.; Ma, Y.-N.; Yao, J.-F.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Bioorg. Chem. 2023, 138, 106674. |
| [18] | Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. J. Med. Chem. 2024, 67, 3885. |
| [19] | Chang, H.-N.; Liu, B.-Y.; Qi, Y.-K.; Zhou, Y.; Chen, Y.-P.; Pan, K.-M.; Li, W.-W.; Zhou, X.-M.; Ma, W.-W.; Fu, C.-Y.; Qi, Y.-M.; Liu, L.; Gao, Y.-F. Angew. Chem. Int. Ed. 2015, 54, 11760. |
| [20] | Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; Zhao, W.; Wu, Y.; Qi, Y.; Liu, L.; Gao, Y. Angew. Chem. Int. Ed. 2020, 59, 15114. |
| [21] | Qi, Y. K.; Zheng, J. S.; Liu, L. Chem 2024, 10, 2390. |
| [22] | Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; Zhao, W.; Wu, Y.; Zhai, W.; Gao, Y. Acta Pharm. Sin. B 2024, 14, 1150. |
| [23] | Vrettos, E. I.; Mezo, G.; Tzakos, A. G. Beilstein J. Org. Chem. 2018, 14, 930. |
| [24] | Alas, M.; Saghaeidehkordi, A.; Kaur, K. J. Med. Chem. 2021, 64, 216. |
| [25] | Lindberg, J.; Nilvebrant, J.; Nygren, P.-A.; Lehmann, F. Molecules 2021, 26, 6042. |
| [26] | Li, Y.-J.; Fang, C.-B.; Wang, S.-S.; Chen, X.-Q.; Li, Y.; Liu, Q.; Qi, Y.-K.; Du, S.-S. Bioorgan. Med. Chem. 2024, 111, 117869. |
| [27] | Gong, L.; Zhao, H.; Liu, Y.; Wu, H.; Liu, C.; Chang, S.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Huang, W. Acta Pharm. Sin. B 2023, 13, 3659. |
| [28] | Hennrich, U.; Kopka, K. Pharmaceuticals 2019, 12, 114. |
| [29] | Strosberg, J. R.; Caplin, M. E.; Kunz, P. L.; Ruszniewski, P. B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E. M.; Yao, J. C.; Pavel, M. E.; Grande, E.; Van Cutsem, E.; Seregni, E.; Duarte, H.; Gericke, G.; Bartalotta, A.; Mariani, M. F.; Demange, A.; Mutevelic, S.; Krenning, E. P.; Investigators, N.-E.-. Lancet Oncol. 2021, 22, 1752. |
| [30] | Griffiths, G. L.; Vasquez, C.; Escorcia, F.; Clanton, J.; Lindenberg, L.; Mena, E.; Choyke, P. L. Adv. Drug Deliv. Rev. 2022, 181, 114086. |
| [31] | Huang, C. M.; Wu, Y. T.; Chen, S. T. Chem. Biol. 2000, 7, 453. |
| [32] | Gaviglio, L.; Gross, A.; Metzler-Nolte, N.; Ravera, M. Metallomics 2012, 4, 260. |
| [33] | Lelle, M.; Kaloyanova, S.; Freidel, C.; Theodoropoulou, M.; Musheev, M.; Niehrs, C.; Stalla, G.; Peneva, K. Mol. Pharm. 2015, 12, 4290. |
| [34] | Ebaston, T. M.; Rozoysky, A.; Zaporozhets, A.; Bazyleyich, A.; Tuchinsky, H.; Marks, V.; Gellerman, G.; Patsenker, L. D. ChemMedChem 2019, 14, 1727. |
| [35] | Rozovsky, A.; Ebaston, T. M.; Zaporozhets, A.; Bazylevich, A.; Tuchinsky, H.; Patsenker, L.; Gellerman, G. RSC Adv. 2019, 9, 32656. |
| [36] | Wang, M.; Liu, J.; Xia, M.; Yin, L.; Zhang, L.; Liu, X.; Cheng, Y. Eur. J. Med. Chem. 2024, 265, 116119. |
| [37] | Zhou, J.; Li, Y.; Huang, W.; Shi, W.; Qian, H. Eur. J. Med. Chem. 2021, 224, 113712. |
| [38] | Deng, X.; Mai, R. Y.; Zhang, C. Y.; Yu, D. B.; Ren, Y. C.; Li, G.; Cheng, B. B.; Li, L.; Yu, Z. Q.; Chen, J. J. Eur. J. Med. Chem. 2021, 213, 113050. |
| [39] | Sharma, A.; Singh, L. R. Eur. J. Med. Chem. 2024, 271, 116456. |
| [40] | Qin, F.; Zhou, H.; Li, J.; Liu, J.; Wang, Y.; Bai, R.; Liu, S.; Manman, M.; Liu, T.; Gao, F.; Du, P.; Lu, X.; Chen, C. Eur. J. Pharmacol. 2021, 905, 174187. |
| [41] | Ding, J.; Wang, T.; Rong, Y.; He, C.; Chen, X. Chinese J. Chem. 2024, 42, 2957. |
| [42] | Huang, Y.; Luo, J.; Li, J.; Zhang, R.; Liu, X.; Fan, Q.; Huang, W. Acta Chim. Sinica 2024, 82, 903. (in Chinese) |
| [42] | (黄艳琴, 罗集文, 李佳启, 张瑞, 刘兴奋, 范曲立, 黄维, 化学学报, 2024, 82, 903.) |
| [43] | Li, M.; Zhang, J.; Liu, L.; Xu, S.; Liu, H. Acta Chim. Sinica 2024, 82, 856. (in Chinese) |
| [43] | (李梦丽, 张婕, 刘丽珍, 徐首红, 刘洪来, 化学学报, 2024, 82, 856.) |
| [44] | Beck, A.; Goetsch, L.; Dumontet, C.; Corva?a, N. Nat. Rev. Drug Discov. 2017, 16, 315. |
| [45] | Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. J. Control. Release 2018, 275, 129. |
| [46] | White, B. H.; Whalen, K.; Kriksciukaite, K.; Alargova, R.; Yeung, T. A.; Bazinet, P.; Brockman, A.; DuPont, M.; Oller, H.; Lemelin, C.-A.; Soo, P. L.; Moreau, B.; Perino, S.; Quinn, J. M.; Sharma, G.; Shinde, R.; Sweryda-Krawiec, B.; Wooster, R.; Bilodeau, M. T. J. Med. Chem. 2019, 62, 2708. |
| [47] | Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Cells 2023, 12, 659. |
| [48] | Lee, J.; Choi, M. K.; Song, I. S. Pharmaceuticals 2023, 16, 802. |
| [49] | Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Mol. Asp. Med. 2023, 93, 101205. |
| [50] | Wang, S.; Sun, L.; Cao, H.; Zhong, Y.; Shao, Z. Acta Chim. Sinica 2021, 79, 1023. (in Chinese) |
| [50] | (王苏杭, 孙灵娜, 曹涵, 钟一鸣, 邵正中, 化学学报, 2021, 79, 1023.) |
| [51] | Guillier, F.; Orain, D.; Bradley, M. Chem. Rev. 2000, 100, 2091. |
| [52] | Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Chem. Rev. 2009, 109, 2455. |
| [53] | Harris, P. W. R.; Kowalczyk, R.; Yang, S.-H.; Williams, G. M.; Brimble, M. A. J. Pept. Sci. 2014, 20, 186. |
| [54] | Skalska, J.; Andrade, V. M.; Cena, G. L.; Harvey, P. J.; Gaspar, D.; Mello, é.; Henriques, S. T.; Valle, J.; Gomes, V. M.; Concei?ao, K.; Castanho, M.; Andreu, D. J. Med. Chem. 2020, 63, 9391. |
| [55] | Wang, J. Y.; Dong, L. Y.; Liu, Y. N.; Chen, X. T.; Ma, Y. N.; Yin, H.; Du, S. S.; Qi, Y. K.; Wang, K. W. Chin. J. Org. Chem. 2021, 41, 2800. (in Chinese) |
| [55] | (王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.) |
| [56] | Chen, X.-T.; Wang, J.-Y.; Ma, Y.-N.; Dong, L.-Y.; Jia, S.-X.; Yin, H.; Fu, X.-Y.; Du, S.-S.; Qi, Y.-K.; Wang, K. J. Pept. Sci. 2022, 28, e3368. |
| [57] | Ma, Y.; Liu, Y. N.; Wang, J.; Chen, X.; Yin, H.; Chi, Q.; Jia, S.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2022, 42, 498. (in Chinese) |
| [57] | (马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 498.) |
| [58] | Yin, H.; Chen, X.; Fu, X.; Ma, Y.; Xu, Y.; Zhang, T.; Liang, S.; Du, S.; Qi, Y.; Wang, K. Acta Chim. Sinica 2022, 80, 444. (in Chinese) |
| [58] | (尹昊, 陈西同, 付邢言, 马艳楠, 徐以梅, 张特, 梁帅, 杜姗姗, 齐昀坤, 王克威, 化学学报, 2022, 80, 444.) |
| [59] | Song, M.; Liu, Q.; Yao, J.-F.; Wang, Y.-T.; Ma, Y.-N.; Xu, H.; Yu, Q.-Y.; Li, Z.; Du, S.-S.; Qi, Y.-K. Bioorgan. Med. Chem. 2024, 107, 117760. |
| [60] | Spears, R. J.; McMahon, C.; Chudasama, V. Chem. Soc. Rev. 2021, 50, 11098. |
| [61] | Pan, M.; Gao, S.; Zheng, Y.; Tan, X.; Lan, H.; Tan, X.; Sun, D.; Lu, L.; Wang, T.; Zheng, Q.; Huang, Y.; Wang, J.; Liu, L. J. Am. Chem. Soc. 2016, 138, 7429. |
| [62] | Zuo, C.; Shi, W.-W.; Chen, X.-X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang, G.-M.; Bierer, D.; Liu, L. Sci. China: Chem. 2019, 62, 1371. |
| [63] | Liang, L.-J.; Chu, G.-C.; Qu, Q.; Zuo, C.; Mao, J.; Zheng, Q.; Chen, J.; Meng, X.; Jing, Y.; Deng, H.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2021, 60, 17171. |
| [64] | Ai, H.; Chu, G.-C.; Gong, Q.; Tong, Z.-B.; Deng, Z.; Liu, X.; Yang, F.; Xu, Z.; Li, J.-B.; Tian, C.; Liu, L. J. Am. Chem. Soc. 2022, 144, 18329. |
| [65] | Ai, H.; Sun, M.; Liu, A.; Sun, Z.; Liu, T.; Cao, L.; Liang, L.; Qu, Q.; Li, Z.; Deng, Z.; Tong, Z.; Chu, G.; Tian, X.; Deng, H.; Zhao, S.; Li, J.-B.; Lou, Z.; Liu, L. Nat. Chem. Biol. 2022, 18, 972. |
| [66] | Zhang, B.; Zheng, Y.; Chu, G.; Deng, X.; Wang, T.; Shi, W.; Zhou, Y.; Tang, S.; Zheng, J.-S.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202306270. |
| [67] | Zhao, R.; Shi, P.; Cui, J.-b.; Shi, C.; Wei, X.-X.; Luo, J.; Xia, Z.; Shi, W.-W.; Zhou, Y.; Tang, J.; Tian, C.; Meininghaus, M.; Bierer, D.; Shi, J.; Li, Y.-M.; Liu, L. Angew. Chem. Int. Ed. 2023, 62, e202216365. |
| [68] | You, Y.; Xu, Z.; Chen, Y. Drug Deliv. 2018, 25, 448. |
| [69] | Randelovic, I.; Schuster, S.; Kapuvari, B.; Fossati, G.; Steinkuehler, C.; Mezo, G.; Tovari, J. Int. J. Mol. Sci. 2019, 20, 4763. |
| [70] | He, Y.; Yuan, X. M.; Lei, P.; Wu, S.; Xing, W.; Lan, X. L.; Zhu, H. F.; Huang, T.; Wang, G. B.; An, R.; Zhang, Y. X.; Shen, G. X. Acta Pharmacol. Sin. 2009, 30, 1053. |
| [71] | Zhang, J.; Jin, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Mol. Pharm. 2010, 7, 1159. |
| [72] | Sanwick, A. M.; Haugh, K. N.; Williams, E. J.; Perry, K. A.; Thiele, N. A.; Chaple, I. F. EJNMMI Radiopharm. Chem. 2024, 9, 88. |
| [73] | Zhou, Y.; Liu, X.; Xue, J.; Liu, L.; Liang, T.; Li, W.; Yang, X.; Hou, X.; Fang, H. J. Med. Chem. 2020, 63, 4701. |
| [74] | Lu, M.; Xing, H.; Shao, W.; Wu, P.; Fan, Y.; He, H.; Barth, S.; Zheng, A.; Liang, X.-J.; Huang, Y. Acta Pharm. Sin. B 2023, 13, 3945. |
| [75] | Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem. Int. Ed. 2011, 50, 7645. |
| [76] | Fang, G.-M.; Wang, J.-X.; Liu, L. Angew. Chem. Int. Ed. 2012, 51, 10347. |
| [77] | Zheng, J.-S.; Tang, S.; Qi, Y.-K.; Wang, Z.-P.; Liu, L. Nat. Protoc. 2013, 8, 2483. |
| [78] | Dong, S.; Zheng, J.-S.; Li, Y.; Wang, H.; Chen, G.; Chen, Y.; Fang, G.; Guo, J.; He, C.; Hu, H.; Li, X.; Li, Y.; Li, Z.; Pan, M.; Tang, S.; Tian, C.; Wang, P.; Wu, B.; Wu, C.; Zhao, J.; Liu, L. Sci. China: Chem. 2024, 67, 1060. |
| [79] | Chi, Q.-N.; Jia, S.-X.; Yin, H.; Wang, L.-E.; Fu, X.-Y.; Ma, Y.-N.; Sun, M.-P.; Qi, Y.-K.; Li, Z.; Du, S.-S. Bioorg. Chem. 2023, 134, 106451. |
| [80] | Kobayashi, K.; Taguchi, A.; Cui, Y.; Shida, H.; Muguruma, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y. Eur. J. Org. Chem. 2021, 2021, 956. |
| [81] | Yin, H.; Fu, X.; Gao, H.; Gao, H.; Ma, Y.; Chen, X.; Zhang, X.; Du, S.-S.; Qi, Y.-K. Front. Oncol. 2023, 12, 1028600. |
| [82] | Qiao, Z.; Qi, H.; Zhang, H.; Zhou, Q.; Wei, N.; Zhang, Y.; Wang, K. Anal. Chem. 2020, 92, 1934. |
/
| 〈 |
|
〉 |