

光诱导醇的含氧衍生物的脱氧/功能化反应的研究进展
 |
赵瑜, 延安大学, 讲师. 2011年本科毕业于咸阳师范学院, 同年考入河南大学化学学院攻读有机化学硕士, 导师为江智勇教授, 于2014年7月获得理学硕士学位. 2015年进入华中师范大学化学学院攻读博士, 导师为肖文精教授和陈加荣教授, 于2018年7月毕业获得有机化学博士学位. 2018年7月进入延安大学化学与化工学院, 从事教学科研工作. 2022年12月进入西北工业大学化工学院从事博后工作, 合作导师为张秋禹教授. 研究方向为自由基反应和光化学合成反应. |
 |
邢彤彤, 男, 汉族, 2002出生于陕西西安. 2024年本科毕业于延安大学, 同年考入延安大学有机化学专业攻读硕士学位, 导师为赵瑜博士. 研究方向为光化学反应促进C-C键的构建. |
 |
段玉荣, 女, 汉族, 1998出生于陕西榆林. 2021年本科毕业于商洛学院, 同年考入延安大学有机化学专业攻读硕士学位, 导师为赵瑜博士. 研究方向为光化学反应促进C-C键的构建.赵全庆, 江苏海洋大学, 副教授. 2014年本科毕业于铜仁学院, 同年考入华中师范大学攻读有机化学硕士学位, 于2020年硕博连读获得博士学位, 导师为肖文精教授和陈加荣教授. 之后, 在德国雷根斯堡大学从事博士后研究, 导师为Oliver Reiser教授. 2023年2月入职江苏海洋大学药学院, 从事教学科研工作. 研究方向为绿色有机合成、药物中间体合成及自由基化学. |
 |
赵全庆, 江苏海洋大学, 副教授. 2014年本科毕业于铜仁学院, 同年考入华中师范大学攻读有机化学硕士学位, 于2020年硕博连读获得博士学位, 导师为肖文精教授和陈加荣教授. 之后, 在德国雷根斯堡大学从事博士后研究, 导师为Oliver Reiser教授. 2023年2月入职江苏海洋大学药学院, 从事教学科研工作. 研究方向为绿色有机合成、药物中间体合成及自由基化学. |
收稿日期: 2025-02-27
网络出版日期: 2025-04-24
基金资助
国家自然科学基金(22161047)
国家自然科学基金(22301109)
江苏海洋大学人才引进项目(KQ23065)
连云港市海燕计划(KK24005)
Recent Advances of Photoinduced Deoxygenative Functionalization of Alcohol Derivatives
Received date: 2025-02-27
Online published: 2025-04-24
Supported by
National Natural Science Foundation of China(22161047)
National Natural Science Foundation of China(22301109)
Research Funds for Talent Introduction of Jiangsu Ocean University(KQ23065)
Lianyungang Haiyan Project(KK24005)
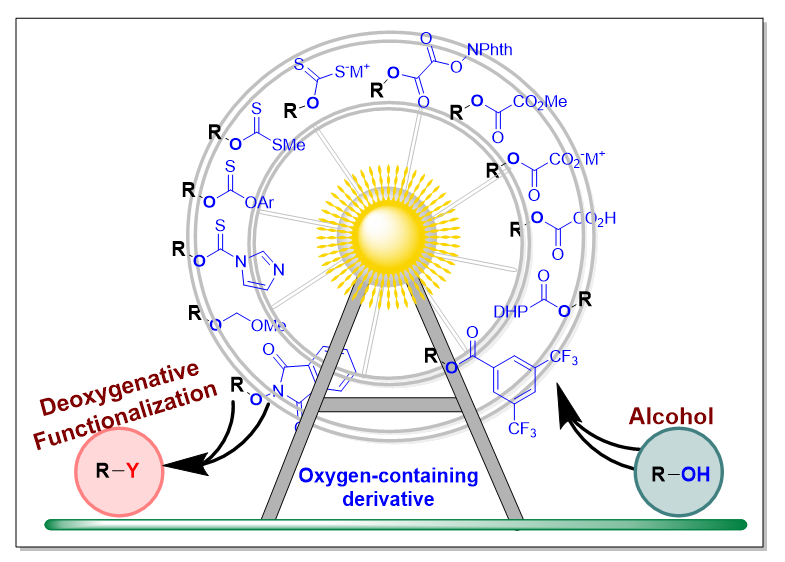
醇类化合物广泛存在于自然界中, 其来源丰富, 是便宜易得的化工原料之一. 早在半个世纪前, Barton- McCombie脱氧反应就被发现, 是研究较为深远的一种醇的脱氧转化方法. 醇的脱氧化反应也是现代有机合成中非常重要的板块之一, 涉及的研究领域相当广泛. 近十几年来, 可见光驱动的氧化还原反应作为一种强劲的有机合成手段, 可以实现许多重要的化学转化, 其具有反应条件温和、官能团兼容性好、反应高效性和环境友好的特点. 对于醇的直接脱氧化反应, 之前已经有文献报道过, 包含可见光促进的光化学反应和过渡金属催化. 在此, 我们主要总结并讨论了从2014年以来, 醇的含氧衍生物, 包括羧酸酯、草酸酯、硫代碳酸酯及其衍生物、醚类和其他醇的含氧衍生物, 在光化学反应中进行的脱氧化/功能化反应的研究进展. 依据醇的含氧衍生物的结构特点进行分类, 详细介绍不同结构类型的醇的含氧衍生物的催化脱氧的反应模式、反应普适性和机理过程等. 展望了该领域所面临的挑战和机遇, 并对其他含羟基结构化合物的光化学脱氧反应具有一定的指导和启发意义.
赵瑜 , 邢彤彤 , 段玉荣 , 赵全庆 . 光诱导醇的含氧衍生物的脱氧/功能化反应的研究进展[J]. 化学学报, 2025 , 83(7) : 773 -802 . DOI: 10.6023/A25020057
Alcohols are one of the most widespread existent in nature, and one of promising chemical feedstocks, due to their cheap and easy availability. Half a century ago, the Barton-McCombie deoxygenation was discovered, and continued to be improved by organic synthetic chemists, becoming an important and rather broad fields in modern organic chemistry. In the last decade, visible-light-driven photoredox catalysis is a powerful tool in organic synthesis, and can realize a good deal of chemical transformations, because of its milder reaction condition, functional group tolerance, high efficiency and environmental-friendly characteristics. It has been reported direct deoxygenation of alcohol in the previous literatures, including the photochemical reactions and metal-transition catalytic systems. Herein, we summarized and discussed some significant advances since in 2014, about the deoxygenative functionalization of alcohol derivatives in photochemical synthetic reactions. And oxygen-containing derivatives of alcohol mainly includes carboxylic esters, oxalic esters, thiocarbonates and their derivatives, ethers and others. In this minireview, in terms of their structural properties, we went to classify and introduce the catalytic modes and mechanisms of deoxygenation, reaction universalities, advantages and shortcomings. In these advances, the oxygen-containing derivatives of alcohol could proceed single electron oxidation and reduction processes, hydrogen atom transfer (HAT), as well as radical addition process, as to assist the C—O bond cleavage to yield the key alkyl radical intermediates, subsequent functionalization to construct the newly C—C, C—N, C—O, C—S, C—X (halo) and C—B bonds. In addition, we also outlooked the challenges and opportunities in the field of deoxygenation of alcohols, and the application prospects of some compounds containing the hydroxy group in the future was discussed as well.

| [1] |
|
| [2] |
|
| [3] |
(a)
(b)
|
| [4] |
(a)
(b)
(c)
|
| [5] |
(a)
(b)
(c)
(d)
(e) Ueng, S-H.;
(f)
(g)
|
| [6] |
|
| [7] |
Recent advances for photoredox catalysis: (a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(李康葵, 龙先扬, 黄岳, 祝诗发, 化学学报, 2024, 82, 658.)
(m)
(归春明, 周潼瑶, 王海峰, 严琼姣, 汪伟, 黄锦, 陈芬儿, 有机化学, 2023, 43, 2647.)
|
| [8] |
(a)
(b)
(c)
(d)
(e)
(f)
(g)
|
| [9] |
Transition metal catalyzed the C—O bond activation: (a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(m)
(n)
袁芳艳, 李超, 罗美明, 曾小明, 化学学报, 2023, 81, 456).
(o)
(p)
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
(a)
(b)
(c)
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(a)
(b)
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
(a)
(b)
|
| [25] |
(a)
(b)
|
| [26] |
(a)
(b)
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
(a)
(b)
(c)
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
(a)
(b)
(c)
(d)
(e)
(f)
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
(a)
(b)
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
(a)
(b)
(c)
(d)
(e)
|
/
| 〈 |
|
〉 |