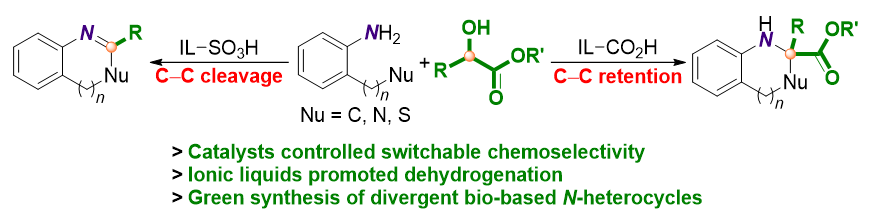
1 引言
2 结果与讨论
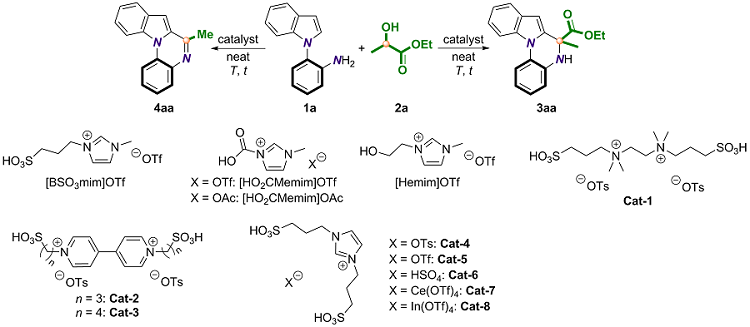
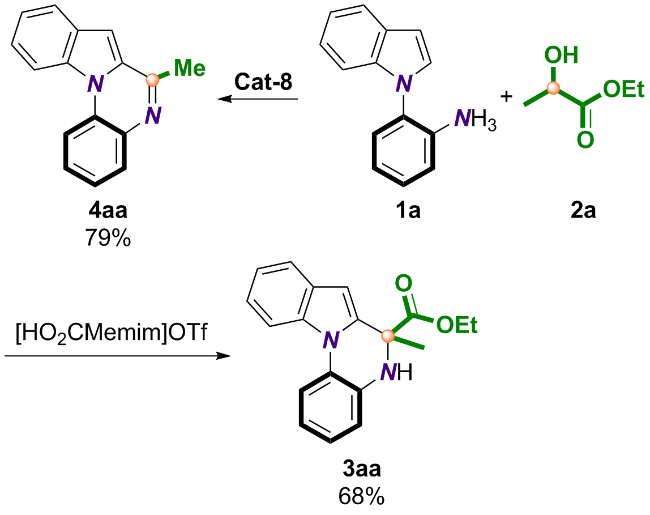
2.1 催化体系的优化
表1 模板底物的反应条件优化aTable 1 Optimization of reaction conditions of model substratesa |
| Entry | Acid (equiv.) | Yield 3aa/% | Yield 4aa/% |
|---|---|---|---|
| 1 | AcOH (1) | 18 | 14 |
| 2 | [BSO3mim]OTf (0.5) | 20 | 40 |
| 3 | [HO2CMemim]OTf (0.5) | 77 | — |
| 4 | [Hemim]OTf (0.5) | 68 | — |
| 5 | Cat-1 (0.5) | — | 56 |
| 6 | Cat-2 (0.5) | 11 | 64 |
| 7 | Cat-3 (0.5) | 7 | 68 |
| 8 | Cat-4 (0.5) | 7 | 76 |
| 9 | Cat-5 (0.5) | 13 | 70 |
| 10 | Cat-6 (0.5) | 9 | 74 |
| 11 | Cat-7 (0.5) | — | 88 |
| 12 | Cat-8 (0.5) | — | 90b |
| 13 | Cat-8 (0.3) | — | 88b (74b,c) |
| 14 | [HO2CMemim]OTf (0.5) | 75 (64c) | — |
| 15 | [HO2CMemim]OAc (0.5) | 71 | — |
| 16 | [HO2CMemim]OTf (0.5) | 72d | 11 |
| 17 | PivOH (1) | 20 | 25 |
| 18 | TsOH (1) | — | 40 |
a Reaction conditions: 1a (0.10 mmol), 2a (0.3 mL), and acid were stirred at 140 ℃ for 12 h under air. b The reaction was conducted for 6 h. c The reaction was carried out at 120 ℃. d The reaction was conducted for 24 h. PivOH: trimethylacetic acid; TsOH: p-toluenesulfonic acid. |
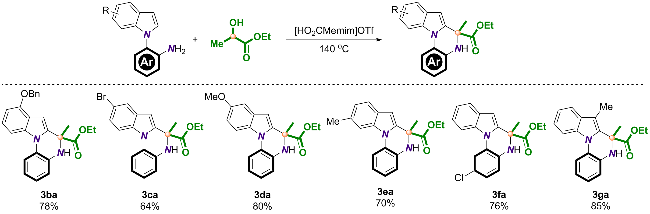
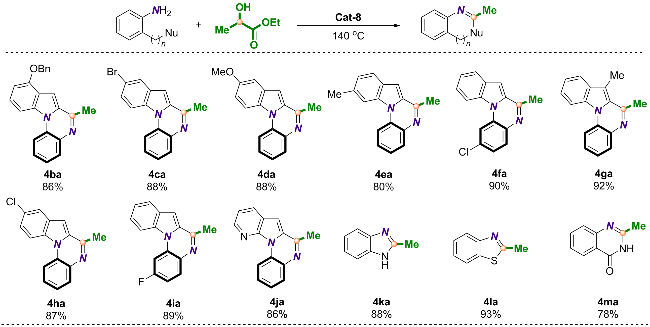
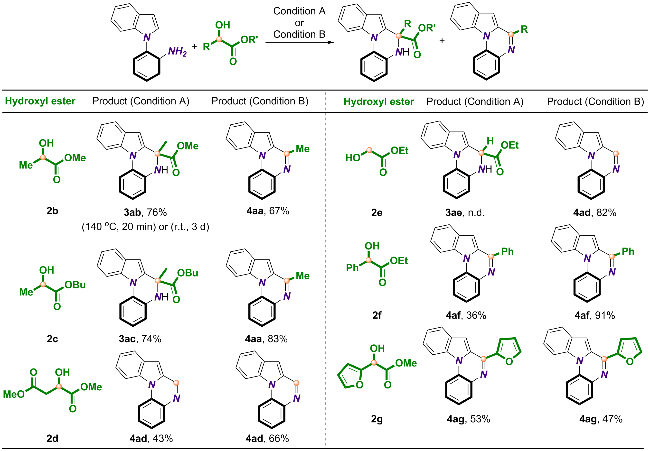
2.2 反应底物的拓展
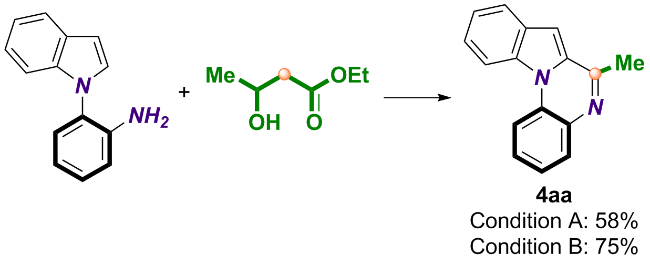
2.3 模板反应的放大
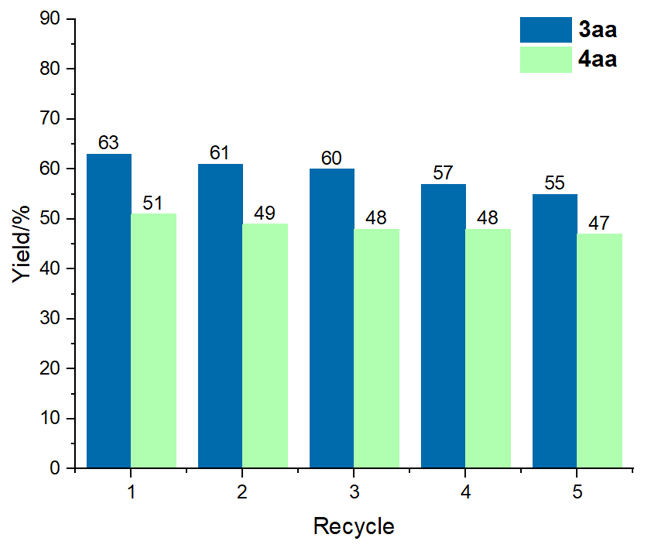
2.4 催化体系的循环性能研究
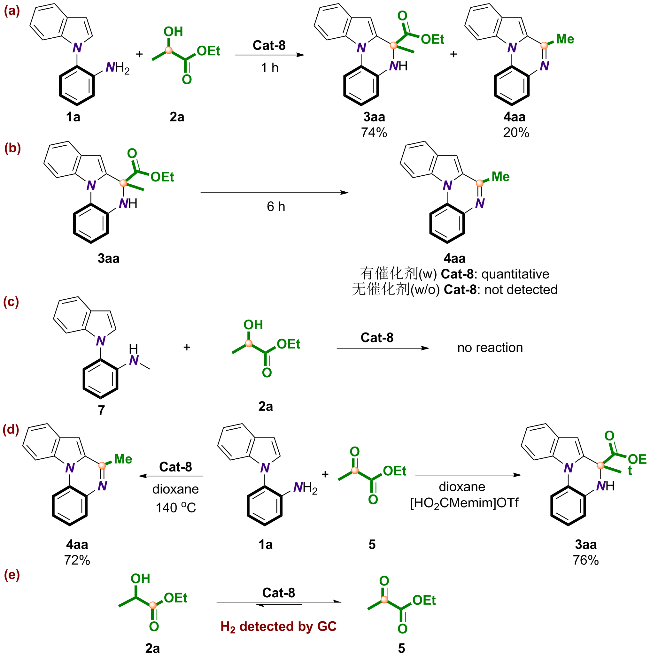
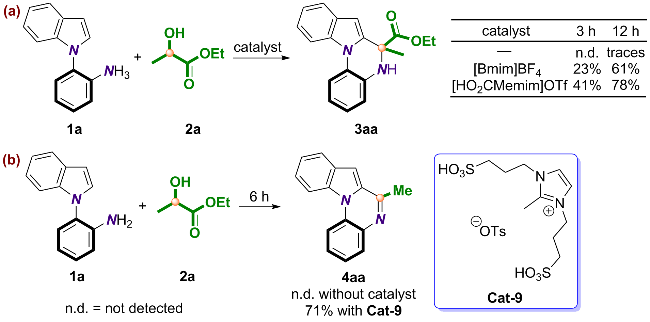
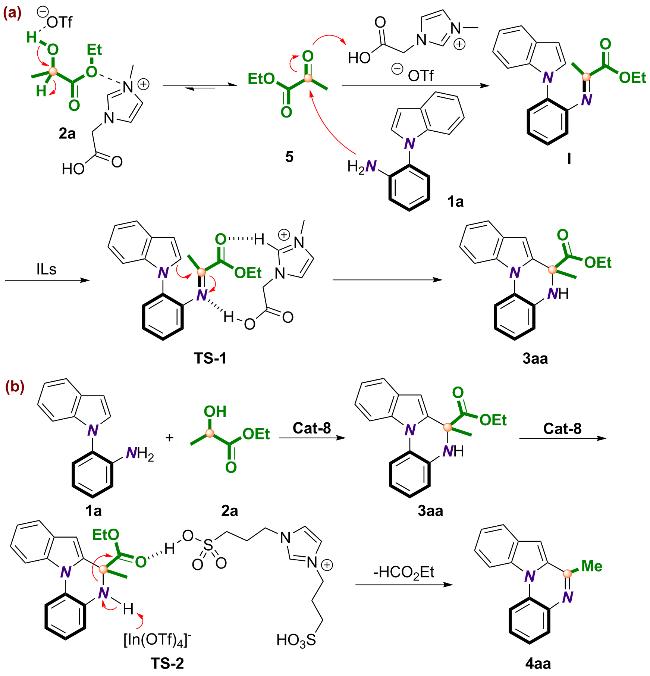
2.5 反应机理的探究
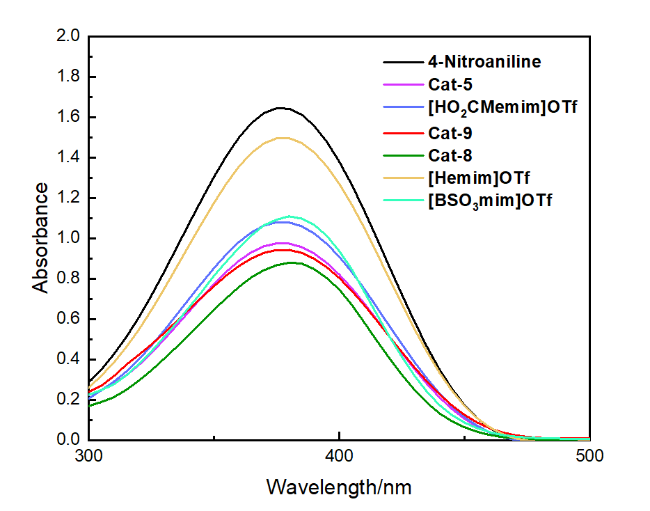
图2 在4-硝基苯胺中测定的离子液体的紫外-可见光谱Figure 2 UV-visible spectra of ILs recorded in 4-nitroaniline |
表2 离子液体的Hammett酸度Table 2 Hammett acidity of ILs |
| Entry | Sample | Amax | [I]/% | [IH+]/% | [I]/[IH+] | H0 |
|---|---|---|---|---|---|---|
| 1 | 4-Nitroaniline | 1.643 | 100 | 0 | — | — |
| 2 | Cat-5 | 0.975 | 59.34 | 40.66 | 1.46 | 1.15 |
| 3 | Cat-8 | 0.875 | 53.25 | 46.75 | 1.14 | 1.05 |
| 4 | Cat-9 | 0.941 | 57.27 | 42.73 | 1.34 | 1.12 |
| 5 | [HO2CMemim]OTf | 1.078 | 65.61 | 34.39 | 1.91 | 1.27 |
| 6 | [Hemim]OTf | 1.459 | 88.80 | 11.20 | 7.93 | 1.89 |
| 7 | [BSO3mim]OTf | 1.087 | 66.15 | 33.85 | 1.95 | 1.28 |