化学学报 ›› 2025, Vol. 83 ›› Issue (5): 479-487.DOI: 10.6023/A25030099 上一篇 下一篇
研究论文
刘珊珊a,*( ), 董微微a, 李珍珍a, 张瑶瑶a, 李超b, 焦林郁c,*(
), 董微微a, 李珍珍a, 张瑶瑶a, 李超b, 焦林郁c,*( )
)
投稿日期:2025-04-01
发布日期:2025-04-28
基金资助:
Shanshan Liua,*( ), Weiwei Donga, Zhenzhen Lia, Yaoyao Zhanga, Chao Lib, Linyu Jiaoc,*(
), Weiwei Donga, Zhenzhen Lia, Yaoyao Zhanga, Chao Lib, Linyu Jiaoc,*( )
)
Received:2025-04-01
Published:2025-04-28
Contact:
* E-mail: Supported by:文章分享
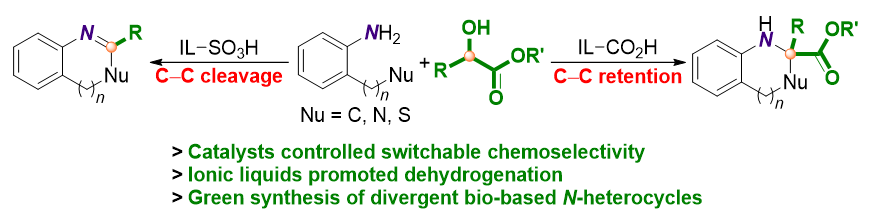
α-羟基酯作为结构可控的碳合成子, 通过离子液体对其反应性的功能性调控, 可以用于一系列N-杂环分子的制备. 具体而言, 含有磺酸官能团的离子液体可以有效催化α-羟基酯与芳香胺的脱氢和脱羧环化反应. 相比之下, 对于羧酸基团取代的离子液体, 许多带有季碳中心的环胺类结构是通过脱氢环化反应制备的, 而其中的脱羧反应通常都会受到抑制. 本研究通过功能化离子液体调控的α-羟基酯的串联脱氢环化反应实现了生物质基N-杂环的可控制备, 所述方案具有转化多样、原料可再生、催化体系绿色且可循环、产物选择性高、底物范围广以及易于放大等诸多优点. 反应机理研究表明, 体系中的氢键效应、阴离子和阳离子的协同性以及酸性官能团等多种作用的结合在促进脱氢、环化以及脱羧过程中表现出多重作用. 尤其重要的是, 两类催化剂的酸性差异是影响产物选择性的首要因素.
刘珊珊, 董微微, 李珍珍, 张瑶瑶, 李超, 焦林郁. 离子液体调控的串联脱氢环化反应多样性合成生物质基氮杂环[J]. 化学学报, 2025, 83(5): 479-487.
Shanshan Liu, Weiwei Dong, Zhenzhen Li, Yaoyao Zhang, Chao Li, Linyu Jiao. Ionic Liquids Controlled Switchable Synthesis of Diverse Bio-Based N-Heterocycles via Tandem Dehydrogenative Cyclization[J]. Acta Chimica Sinica, 2025, 83(5): 479-487.
| Entry | Acid (equiv.) | Yield 3aa/% | Yield 4aa/% |
|---|---|---|---|
| 1 | AcOH (1) | 18 | 14 |
| 2 | [BSO3mim]OTf (0.5) | 20 | 40 |
| 3 | [HO2CMemim]OTf (0.5) | 77 | — |
| 4 | [Hemim]OTf (0.5) | 68 | — |
| 5 | Cat-1 (0.5) | — | 56 |
| 6 | Cat-2 (0.5) | 11 | 64 |
| 7 | Cat-3 (0.5) | 7 | 68 |
| 8 | Cat-4 (0.5) | 7 | 76 |
| 9 | Cat-5 (0.5) | 13 | 70 |
| 10 | Cat-6 (0.5) | 9 | 74 |
| 11 | Cat-7 (0.5) | — | 88 |
| 12 | Cat-8 (0.5) | — | 90b |
| 13 | Cat-8 (0.3) | — | 88b (74b,c) |
| 14 | [HO2CMemim]OTf (0.5) | 75 (64c) | — |
| 15 | [HO2CMemim]OAc (0.5) | 71 | — |
| 16 | [HO2CMemim]OTf (0.5) | 72d | 11 |
| 17 | PivOH (1) | 20 | 25 |
| 18 | TsOH (1) | — | 40 |
| Entry | Acid (equiv.) | Yield 3aa/% | Yield 4aa/% |
|---|---|---|---|
| 1 | AcOH (1) | 18 | 14 |
| 2 | [BSO3mim]OTf (0.5) | 20 | 40 |
| 3 | [HO2CMemim]OTf (0.5) | 77 | — |
| 4 | [Hemim]OTf (0.5) | 68 | — |
| 5 | Cat-1 (0.5) | — | 56 |
| 6 | Cat-2 (0.5) | 11 | 64 |
| 7 | Cat-3 (0.5) | 7 | 68 |
| 8 | Cat-4 (0.5) | 7 | 76 |
| 9 | Cat-5 (0.5) | 13 | 70 |
| 10 | Cat-6 (0.5) | 9 | 74 |
| 11 | Cat-7 (0.5) | — | 88 |
| 12 | Cat-8 (0.5) | — | 90b |
| 13 | Cat-8 (0.3) | — | 88b (74b,c) |
| 14 | [HO2CMemim]OTf (0.5) | 75 (64c) | — |
| 15 | [HO2CMemim]OAc (0.5) | 71 | — |
| 16 | [HO2CMemim]OTf (0.5) | 72d | 11 |
| 17 | PivOH (1) | 20 | 25 |
| 18 | TsOH (1) | — | 40 |

| Entry | Sample | Amax | [I]/% | [IH+]/% | [I]/[IH+] | H0 |
|---|---|---|---|---|---|---|
| 1 | 4-Nitroaniline | 1.643 | 100 | 0 | — | — |
| 2 | Cat-5 | 0.975 | 59.34 | 40.66 | 1.46 | 1.15 |
| 3 | Cat-8 | 0.875 | 53.25 | 46.75 | 1.14 | 1.05 |
| 4 | Cat-9 | 0.941 | 57.27 | 42.73 | 1.34 | 1.12 |
| 5 | [HO2CMemim]OTf | 1.078 | 65.61 | 34.39 | 1.91 | 1.27 |
| 6 | [Hemim]OTf | 1.459 | 88.80 | 11.20 | 7.93 | 1.89 |
| 7 | [BSO3mim]OTf | 1.087 | 66.15 | 33.85 | 1.95 | 1.28 |
| Entry | Sample | Amax | [I]/% | [IH+]/% | [I]/[IH+] | H0 |
|---|---|---|---|---|---|---|
| 1 | 4-Nitroaniline | 1.643 | 100 | 0 | — | — |
| 2 | Cat-5 | 0.975 | 59.34 | 40.66 | 1.46 | 1.15 |
| 3 | Cat-8 | 0.875 | 53.25 | 46.75 | 1.14 | 1.05 |
| 4 | Cat-9 | 0.941 | 57.27 | 42.73 | 1.34 | 1.12 |
| 5 | [HO2CMemim]OTf | 1.078 | 65.61 | 34.39 | 1.91 | 1.27 |
| 6 | [Hemim]OTf | 1.459 | 88.80 | 11.20 | 7.93 | 1.89 |
| 7 | [BSO3mim]OTf | 1.087 | 66.15 | 33.85 | 1.95 | 1.28 |
| [1] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [2] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [3] |
(a)
|
|
(b)
|
|
| [4] |
(a)
pmid: 31039284 |
|
(b)
doi: 10.1002/cssc.201900928 pmid: 31039284 |
|
| [5] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(杨帆, 方婷, 杨桂春, 高梦, 有机化学, 2024, 44, 1021).
doi: 10.6023/cjoc202307002 |
|
|
(e)
|
|
|
(罗江浩, 马浩文, 张杰豪, 周伟, 蔡倩, 化学学报, 2023, 81, 898).
doi: 10.6023/A23040164 |
|
| [6] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [7] |
(a)
doi: 10.1021/acscatal.7b03843 pmid: 29527403 |
|
(b)
pmid: 29527403 |
|
| [8] |
(a)
|
|
(b)
|
|
| [9] |
doi: 10.1038/s41467-020-18532-3 pmid: 32994420 |
| [10] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(谭芳芳, 史孟欣, 张文敏, 李洋, 有机化学, 2025, 10.6023/cjoc202408018).
|
|
| [11] |
(a)
|
|
(b)
|
|
| [12] |
|
| [13] |
(a)
pmid: 28266216 |
|
(b)
doi: 10.1021/acs.chemrev.6b00516 pmid: 28266216 |
|
|
(c)
pmid: 28266216 |
|
|
(d)
pmid: 28266216 |
|
| [14] |
(a)
doi: 10.1021/acs.joc.8b02509 pmid: 30511574 |
|
(b)
pmid: 30511574 |
|
|
(c)
pmid: 30511574 |
|
|
(d)
pmid: 30511574 |
|
| [15] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [16] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(鞠嘉浩, 徐吉磊, 王康军, 黄家辉, 化学学报, 2024, 82, 1216).
doi: 10.6023/A24080246 |
|
|
(d)
|
|
|
(曾少娟, 孙雪琦, 白银鸽, 白璐, 郑爽, 张香平, 张锁江, 化学学报, 2023, 81, 627).
doi: 10.6023/A23030063 |
|
|
(e)
|
|
|
(刘思洁, 陆燕玲, 黄家荣, 高梦翘, 龙金星, 李雪辉, 中国科学(化学), 2021, 51, 1382).
|
|
| [17] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
| [18] |
(a)
doi: 10.1021/jm501100b pmid: 25255204 |
|
(b)
pmid: 25255204 |
|
|
(赵玉洁, 焦林郁, 有机化学, 2025, 45, 1040).
doi: 10.6023/cjoc202500007 pmid: 25255204 |
|
| [19] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [20] |
(a)
pmid: 12720424 |
|
(b)
pmid: 12720424 |
| [1] | 李菲, 伊思静, 王姣, 刘晓霞, 高文梅, 李婧婧, 张海峰. 稀土超分子荧光有序聚集体在离子液体中聚集及荧光性能的研究[J]. 化学学报, 2025, 83(5): 453-462. |
| [2] | 贺乾军, 张晨杰, 徐敏敏, 袁亚仙, 姚建林. 电化学SERS研究离子液体/金属界面水吸附行为的阳离子亲水性效应[J]. 化学学报, 2024, 82(12): 1202-1208. |
| [3] | 鞠嘉浩, 徐吉磊, 王康军, 黄家辉. 果糖一步法制备5-甲氧基甲基-2-呋喃甲醛及树脂催化剂再生方法探究[J]. 化学学报, 2024, 82(12): 1216-1225. |
| [4] | 曾少娟, 孙雪琦, 白银鸽, 白璐, 郑爽, 张香平, 张锁江. CO2捕集分离的功能离子液体及材料研究进展★[J]. 化学学报, 2023, 81(6): 627-645. |
| [5] | 刘稳, 王昱捷, 杨慧琴, 李成杰, 吴娜, 颜洋. 离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极[J]. 化学学报, 2023, 81(10): 1379-1386. |
| [6] | 李晓倩, 张靖, 苏芳芳, 王德超, 姚东东, 郑亚萍. 多孔离子液体的构筑及应用[J]. 化学学报, 2022, 80(6): 848-860. |
| [7] | 武文俊, 李玉婷, 冯茜, 丁文星. 钙钛矿双功能钝化剂: 室温离子液体的机械化学制备[J]. 化学学报, 2022, 80(11): 1469-1475. |
| [8] | 王赫男, 张安歌, 张仲, 田洪瑞, 岳倩, 赵雪, 鹿颖, 刘术侠. 基于稀土阳离子和多酸阴离子的系列纯无机离子液体的合成及性质[J]. 化学学报, 2021, 79(7): 920-924. |
| [9] | 吕玉苗, 陈伟, 王艳磊, 霍锋, 董依慧, 魏莉, 何宏艳. 离子液体二维结构制备及其特性研究进展[J]. 化学学报, 2021, 79(4): 443-458. |
| [10] | 王引航, 李伟, 罗沙, 刘守新, 马春慧, 李坚. 离子液体固载型功能材料的应用研究进展[J]. 化学学报, 2018, 76(2): 85-94. |
| [11] | 邱华玉, 赵井文, 周新红, 崔光磊. 离子液体-无机颗粒杂化电解质在二次电池中的研究进展[J]. 化学学报, 2018, 76(10): 749-756. |
| [12] | 张川, 张鲁嘉, 张洋, 黄和, 胡燚. 基于分子模拟的离子液体修饰Porcine Pancreas脂肪酶催化性能和稳定性的相关研究[J]. 化学学报, 2016, 74(1): 74-80. |
| [13] | 何学侠, 刘富才, 曾庆圣, 刘政 . 二维材料双电层场晶体管的研究[J]. 化学学报, 2015, 73(9): 924-935. |
| [14] | 冷明浩, 陈仕谋, 张军玲, 郎海燕, 康艳红, 张锁江. 含羰基有机添加剂对AlCl3-[Emim]Cl电沉积铝的影响[J]. 化学学报, 2015, 73(5): 403-408. |
| [15] | 钱文静, 袁超, 郭江娜, 严锋. 聚离子液体功能材料研究进展[J]. 化学学报, 2015, 73(4): 310-315. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||