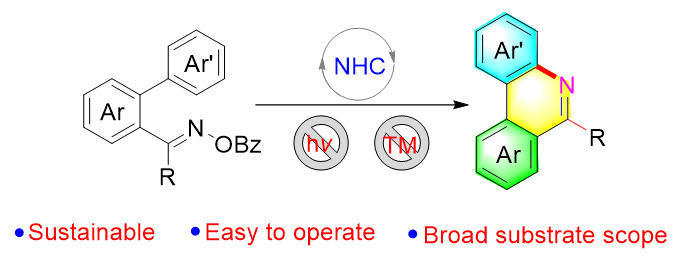
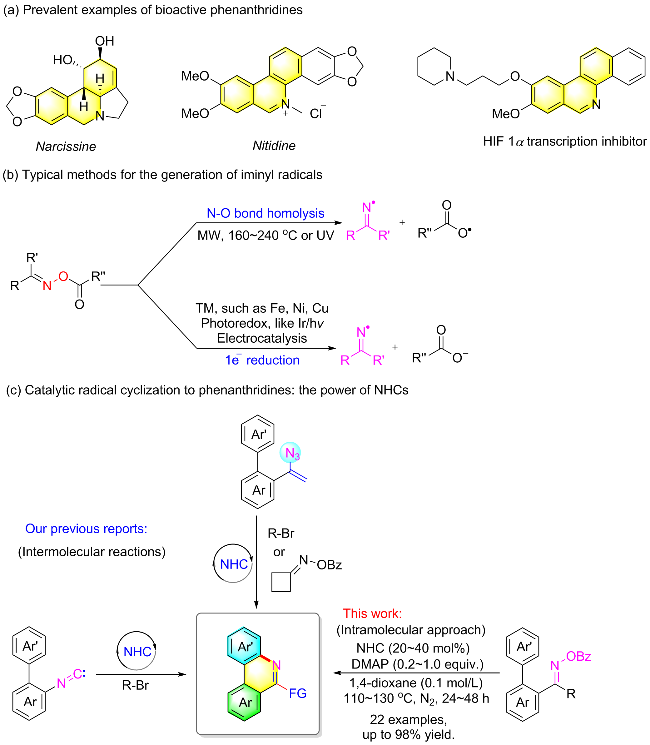
1 引言
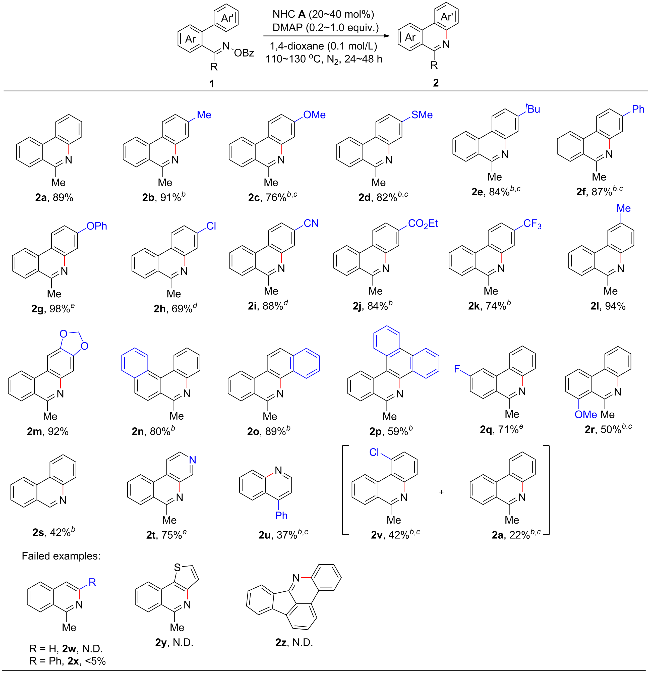
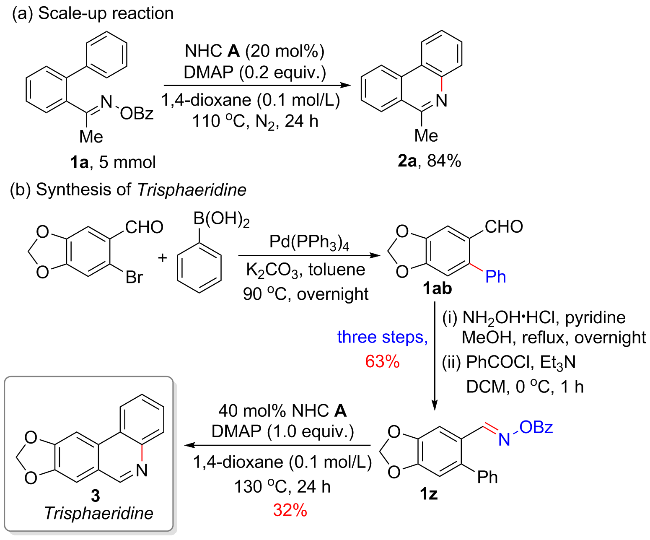
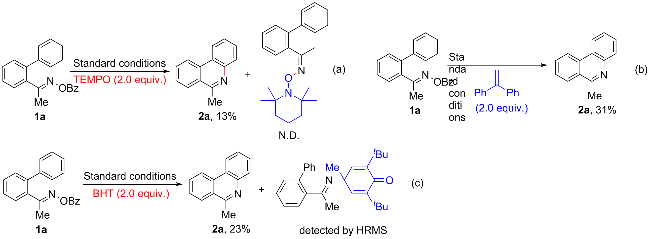
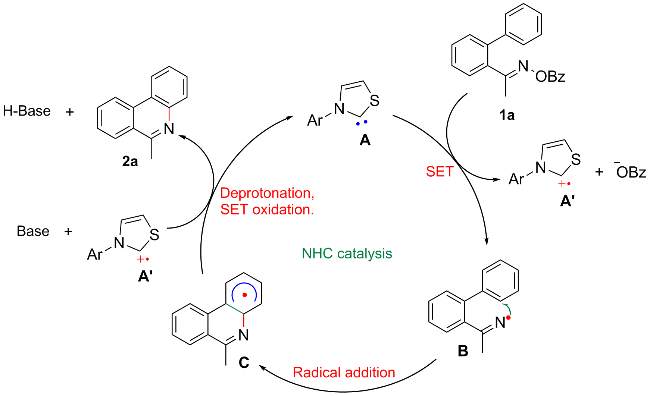
2 结果与讨论
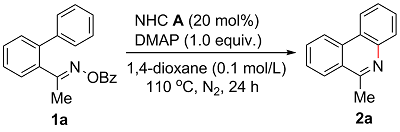
2.1 反应条件优化
表1 反应条件优化Table 1 Reaction conditions optimization |
| Entry | Variations | Yielda/% |
|---|---|---|
| 1 | None | 88 |
| 2 | MeCN as the solvent | 70 |
| 3 | PhOMe as the solvent | 35 |
| 4 | DMSO as the solvent | 76 |
| 5 | K3PO4 as the base | 19 |
| 6 | tBuONa as the base | 25 |
| 7 | DBU as the base | 58 |
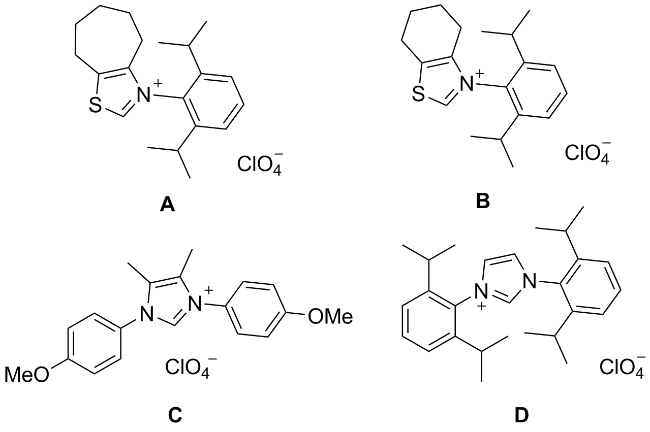
| 8 | NHC B as the catalyst | 76 |
| 9 | NHC C as the catalyst | 22 |
| 10 | NHC D as the catalyst | 57 |
| 11 | 0.2 equiv. of DMAP | 90 |
| 12b | React for 8 h | 40 |
| 13 | Without NHC A | N.D. |
a Reaction conditions: 1a (0.1 mmol), NHC A (0.02 mmol), DMAP (0.1 mmol), 1,4-dioxane (1.0 mL), 110 ℃, N2, 24 h, NMR yield; b DMAP (0.02 mmol); N.D.: Not detected. DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene. |