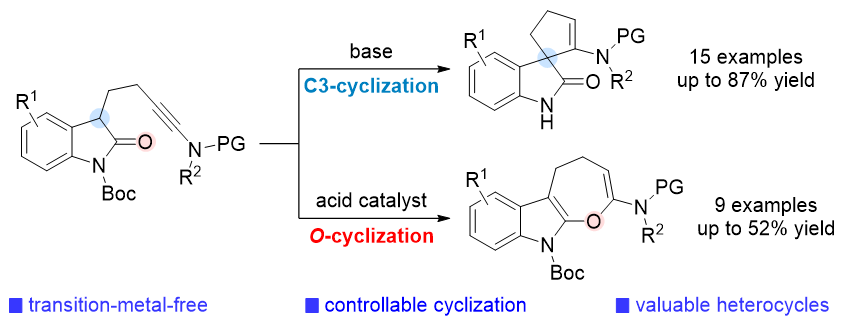
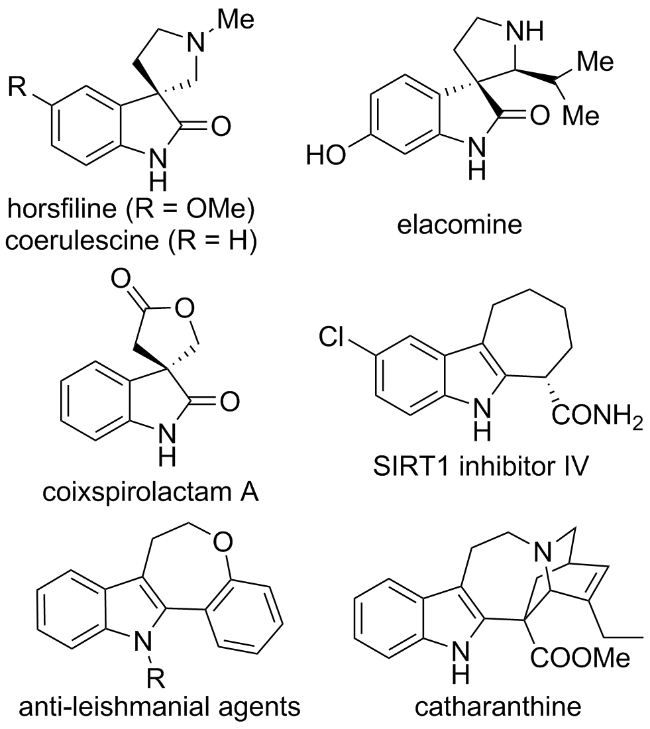
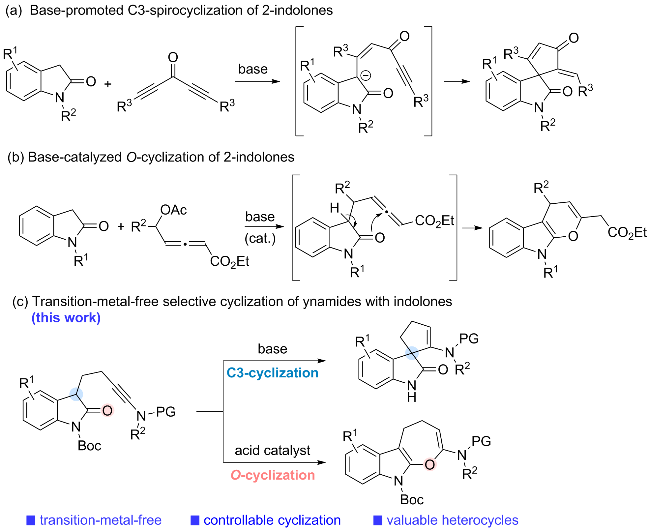
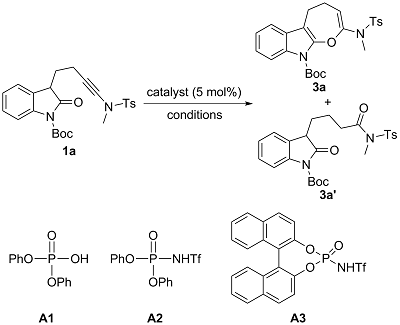
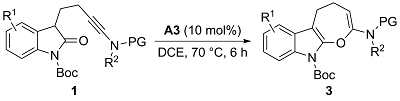
1 引言
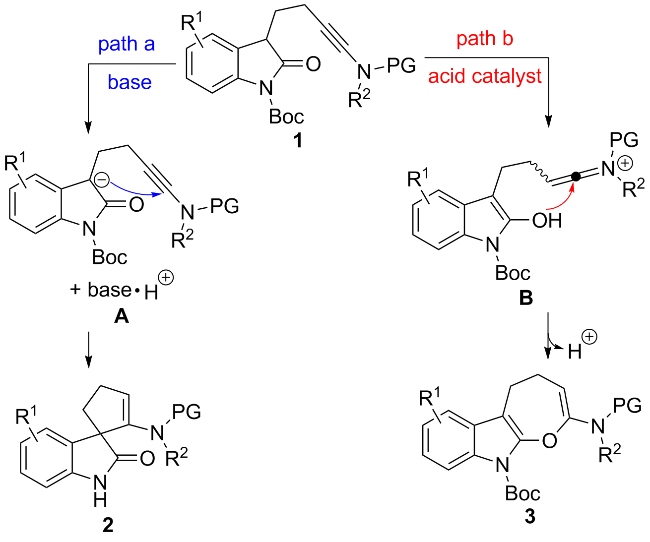
2 结果与讨论
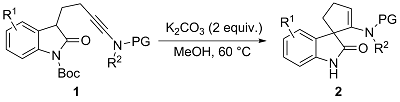
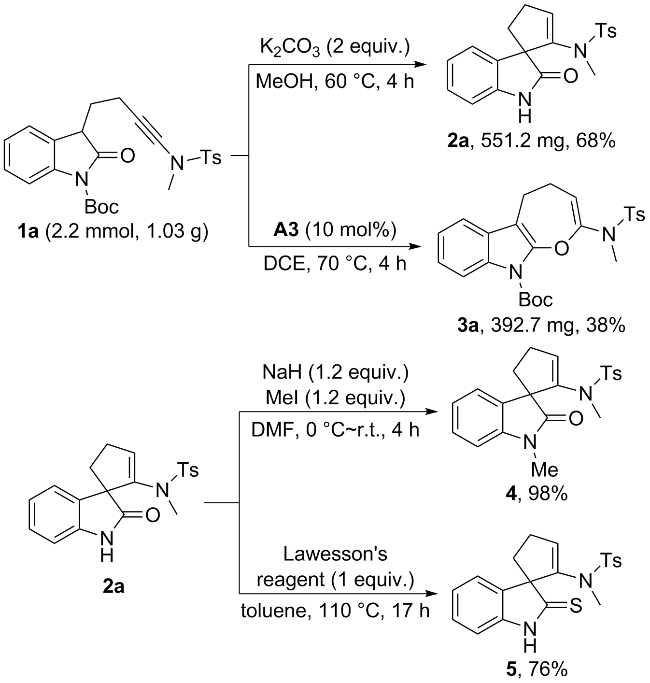
2.1 碱促进的反应条件优化及底物普适性考察
表1 碱促进的螺环吲哚酮化合物2a的合成条件优化aTable 1 Reaction condition optimization for the base-promoted synthesis of spiroindolone 2aa |
| Entry | Base | Conditions | Yieldb/% |
|---|---|---|---|
| 1 | Et3N | MeOH, 60 ℃, 14 h | <5 |
| 2 | DBU | MeOH, 60 ℃, 14 h | <5 |
| 3 | NaH | MeOH, 60 ℃, 6 h | 53 |
| 4 | KOH | MeOH, 60 ℃, 6 h | 59 |
| 5 | K3PO4 | MeOH, 60 ℃, 6 h | 65 |
| 6 | Na2CO3 | MeOH, 60 ℃, 6 h | 40 |
| 7 | Cs2CO3 | MeOH, 60 ℃, 6 h | 45 |
| 8 | K2CO3 | MeOH, 60 ℃, 6 h | 67 |
| 9 | K2CO3 | DCE, 60 ℃, 6 h | <5 |
| 10 | K2CO3 | THF, 60 ℃, 6 h | <5 |
| 11 | K2CO3 | PhMe, 60 ℃, 6 h | <5 |
| 12 | K2CO3 | DMF, 60 ℃, 6 h | 39 |
| 13 | K2CO3 | MeOH, 50 ℃, 6 h | 53 |
| 14c | K2CO3 | MeOH, 60 ℃, 6 h | 82 |
a Reaction conditions: 1a (0.025 mmol), base (0.0375 mmol), solvent (0.5 mL), 50~60 ℃, 6~14 h, in vials. b Measured by 1H NMR using 1,3,5-trimethoxyl- benzene as the internal standard. c K2CO3 (2 equiv.) was added. |
表2 碱促进的炔酰胺1的螺环化反应底物范围研究aTable 2 Substrate scope study for the base-promoted spirocyclization of ynamides 1a |
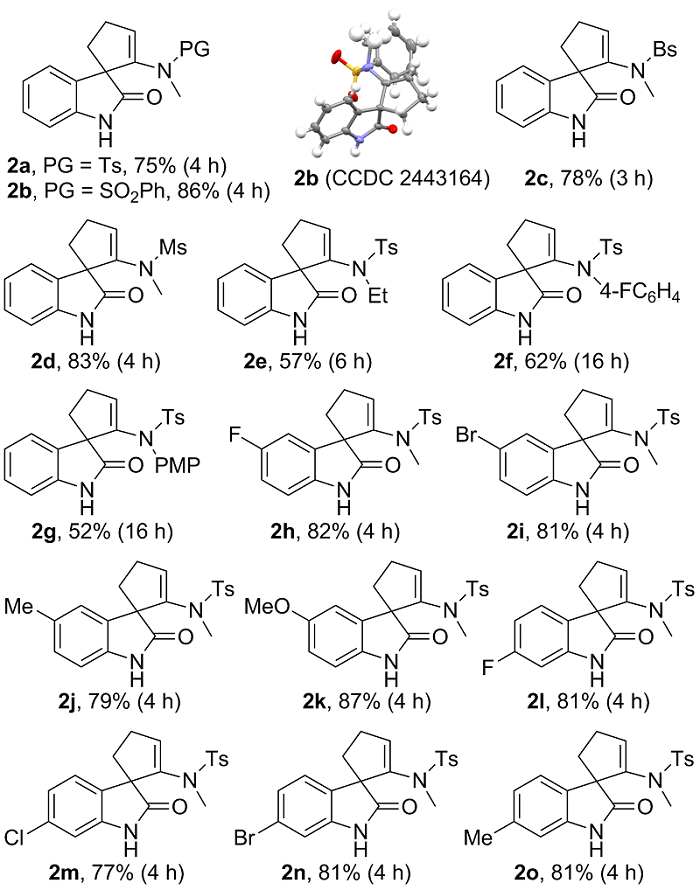 |
a Reaction conditions: 1 (0.2 mmol), K2CO3 (0.4 mmol), MeOH (4 mL), 60 ℃, 3~16 h, in vials; yields are those for the isolated products. PG=protecting group. Bs=4-bromobenzenesulfonyl. Ms=methanesulfonyl. PMP=4-methoxyphenyl. |
2.2 酸催化的反应条件优化及底物普适性考察
表3 酸催化的吲哚并七元环化合物3a的合成条件优化aTable 3 Reaction condition optimization for the acid-catalyzed synthesis of indole-fused seven-membered ring 3aa |
| Entry | Catalyst | Conditions | Yieldb/% | |
|---|---|---|---|---|
| 3a | 3a' | |||
| 1 | MsOH | DCE, 70 ℃, 6 h | <5 | 37 |
| 2 | TsOH | DCE, 70 ℃, 6 h | <5 | 43 |
| 3 | HNTf2 | DCE, 70 ℃, 0.5 h | <5 | 77 |
| 4 | TfOH | DCE, 70 ℃, 1 h | <5 | 84 |
| 5 | A1 | DCE, 70 ℃, 8 h | <5 | 65 |
| 6 | A2 | DCE, 70 ℃, 8 h | <5 | 68 |
| 7 | A3 | DCE, 70 ℃, 6 h | 30 | 12 |
| 8c | A3 | DCE, 70 ℃, 6 h | 48 | 9 |
a Reaction conditions: 1a (0.025 mmol), catalyst (0.00125 mmol), solvent (0.5 mL), 70 ℃, 0.5~8 h, in vials. b Measured by 1H NMR using 1,3,5-tri- methoxylbenzene as the internal standard. c A3 (0.0025 mmol) was used. |
表4 酸催化的炔酰胺1的选择性环化反应底物范围研究aTable 4 Substrate scope study for the acid-catalyzed selective cyclization of ynamides 1a |
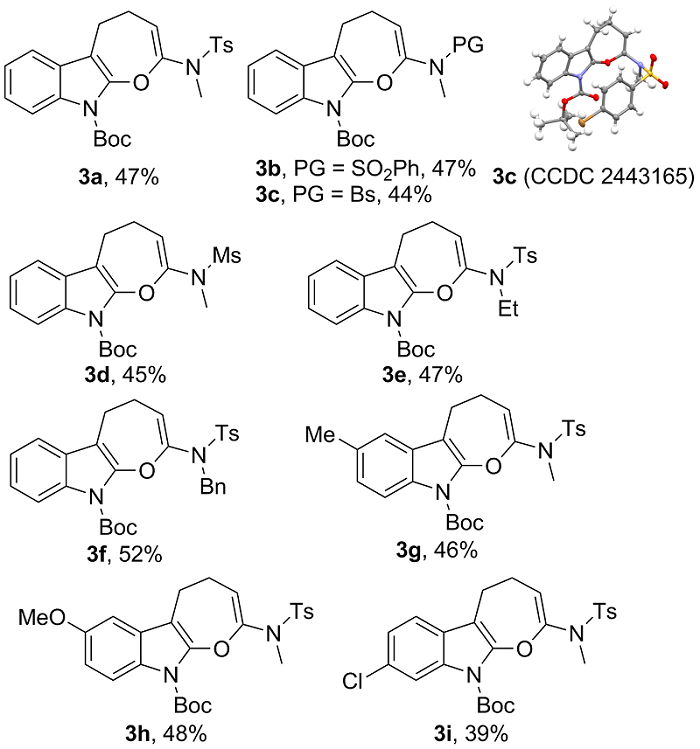 |
a Reaction conditions: 1 (0.2 mmol), A3 (0.02 mmol), DCE (4 mL), 70 ℃, 6 h, in vials; yields are those for the isolated products. |