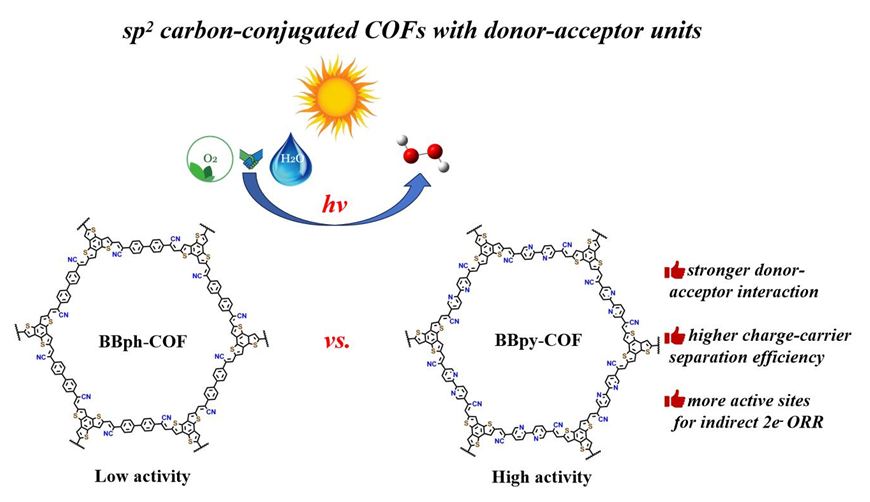
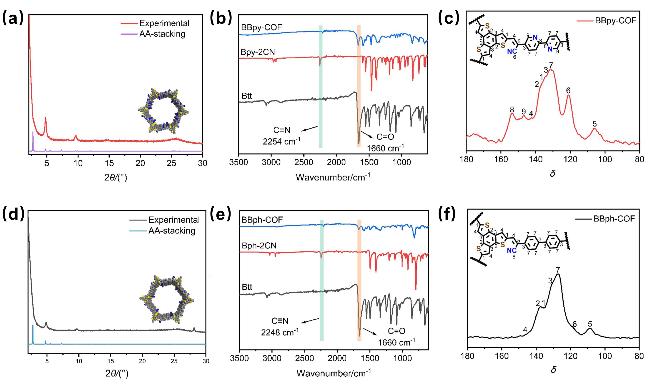
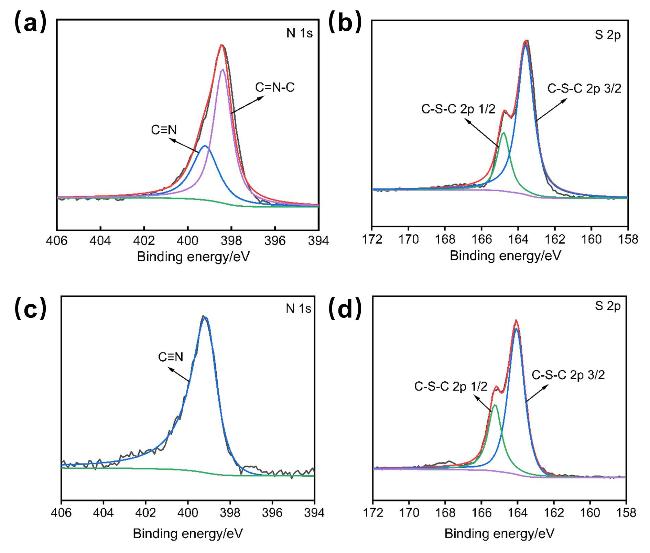
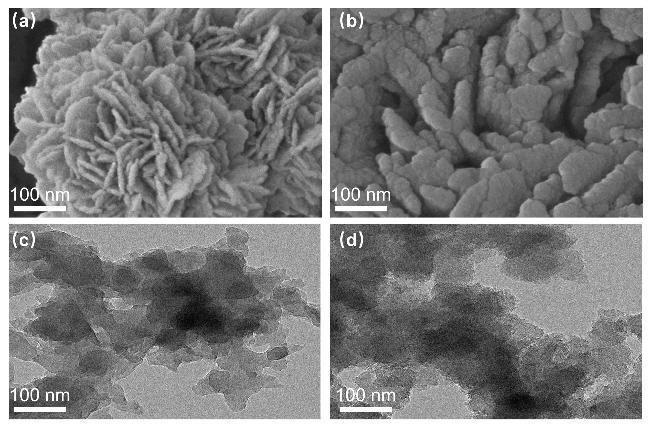
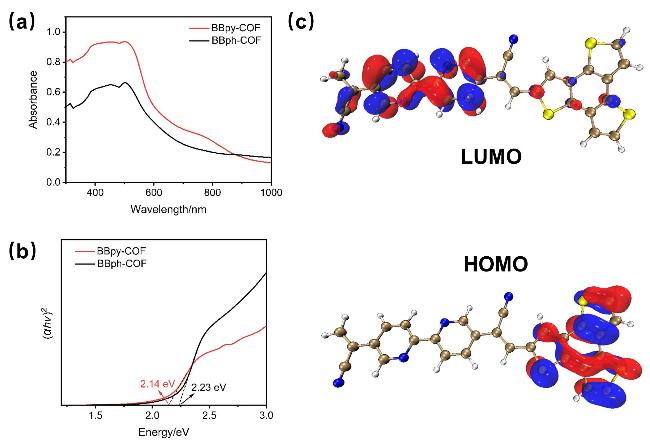
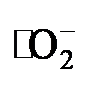作为一种经典的
N-杂环共轭化合物, 联吡啶(Bpy)作为光催化氧还原反应(ORR)的活性位点, 可提升COFs光催化产H
2O
2的性能
[20]. 跟其它光催化剂一样, COFs也存在光生载流子分离效率低下的通病, 构筑电子给体-受体(D-A)型COFs是调控光生载流子分离和输运性能的有效策略
[21], 其关键在于D-A有效配对. 鉴于Bpy单元本身又具有缺电子的结构特性, 可充当电子受体
[22], 因此, 课题组选定2,2'-([2'-联吡啶]-5,5'-二基)二乙腈(Bpy-2CN)连接体扮演电子受体“角色”. 与之同时, 课题组前期研究发现, 具有
C3h对称性的平面共轭系统和富含硫的苯并[1,2-
b:3,4-
b':5,6-
b'']三噻吩-2,5,8-三醛(Btt)具有优异的化学稳定性和光电化学活性, 且该单元内部的3个噻吩环混合在苯环中心, 可有效地促进π电子的共轭, Btt与电子受体2,2'-(苯并[
c][
1,
2,
5]噻二唑- 4,7-二基双(4,1-亚苯基))二乙腈(Bdd)构筑D-A型Btt-Bdd-COF, 显著增强光催化有机转化性能
[23]. 基于此, 有效应用“三合一”集成策略, 以富电子Btt为电子供体, 与强电子受体和ORR活性中心Bpy有效配对, 构筑sp
2-碳共轭D-A结构BBpy-COF, 在无牺牲剂体系中开展光催化纯水和氧气制备H
2O
2的研究. 为进行对比研究, 又分别以Btt和联苯(Bph)为电子供体和弱电子受体
[24], 制备对比材料BBph-COF. 本项工作将为合理设计和制备高活性和稳定性的sp
2-碳共轭D-A结构COFs提供借鉴和参考.
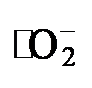 中间体是热力学可行的, 而
中间体是热力学可行的, 而 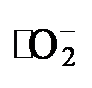 中间体可进一步转化为H2O2[37].
中间体可进一步转化为H2O2[37].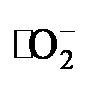 的捕获剂(图6d). 引入t-BA对光催化产H2O2影响较小, 说明•OH不是光催化ORR的中间体. 然而, 通过添加C2H5OH来捕获空穴, 其为光催化ORR提供了更多的光生电子, 进而提高光合成H2O2的生成速率. 值得注意的是, 在AgNO3或p-BQ存在下, 光催化合成H2O2生成速率被显著抑制, 这表明光催化产H2O2的主要活性种是e−和
的捕获剂(图6d). 引入t-BA对光催化产H2O2影响较小, 说明•OH不是光催化ORR的中间体. 然而, 通过添加C2H5OH来捕获空穴, 其为光催化ORR提供了更多的光生电子, 进而提高光合成H2O2的生成速率. 值得注意的是, 在AgNO3或p-BQ存在下, 光催化合成H2O2生成速率被显著抑制, 这表明光催化产H2O2的主要活性种是e−和 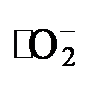 . 简言之, BBpy-COF自由基淬灭实验证明了光催化ORR为两步单电子路线(O2还原为
. 简言之, BBpy-COF自由基淬灭实验证明了光催化ORR为两步单电子路线(O2还原为 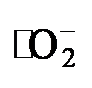 , 再还原为H2O2)[24].
, 再还原为H2O2)[24].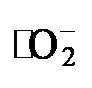 信号, 而在光照射5 min后, 则检测到DMPO-
信号, 而在光照射5 min后, 则检测到DMPO- 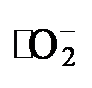 的强烈共振信号, 表明在光催化合成H2O2过程中产生了更多的
的强烈共振信号, 表明在光催化合成H2O2过程中产生了更多的 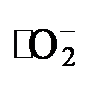 自由基[43]. 此外, 由图6f可见(见支持信息图S11), 通过Koutecky-Levich方法拟合曲线计算出BBpy-COF的平均转移电子数(n)为2.27, 其接近两电子ORR的理论值(n=2), 进一步证明了BBpy-COF在可见光照射下主要通过两电子ORR途径生成H2O2[44]. 综上所述, 结合能带结构、不同条件下对照实验、自由基淬灭实验、EPR光谱和ORR的n值测定, 确认了BBpy-COF在可见光照射下由纯水和氧气通过两步单电子ORR生成H2O2[45].
自由基[43]. 此外, 由图6f可见(见支持信息图S11), 通过Koutecky-Levich方法拟合曲线计算出BBpy-COF的平均转移电子数(n)为2.27, 其接近两电子ORR的理论值(n=2), 进一步证明了BBpy-COF在可见光照射下主要通过两电子ORR途径生成H2O2[44]. 综上所述, 结合能带结构、不同条件下对照实验、自由基淬灭实验、EPR光谱和ORR的n值测定, 确认了BBpy-COF在可见光照射下由纯水和氧气通过两步单电子ORR生成H2O2[45].