1 引言
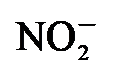 还原为NH3. Yang等[21]制备了CoP@Co核壳催化剂, 研究发现通过改变催化剂结构可以优化催化剂的合成氨反应性能. 上述研究表明, 钴基催化剂适用于NRR反应.
还原为NH3. Yang等[21]制备了CoP@Co核壳催化剂, 研究发现通过改变催化剂结构可以优化催化剂的合成氨反应性能. 上述研究表明, 钴基催化剂适用于NRR反应.
具有内置电场效应的Cu2S@Co3S4/CF核壳异质结催化剂氮还原性能研究
收稿日期: 2025-03-24
网络出版日期: 2025-06-20
基金资助
国家自然科学基金有序能量转换基础科学中心项目(52488201)
Nitrogen Reduction Properties of Cu2S@Co3S4/CF Core-shell Heterojunction Catalysts with Built-in Electric Field Effect
Received date: 2025-03-24
Online published: 2025-06-20
Supported by
Ordered Energy Conversion Basic Science Center Project of the National Natural Science Foundation of China(52488201)
在能源危机和可持续发展的背景下, 具有零碳排放特性的氮还原合成氨(NRR)方法具有很好的前景. 开发具有高氨产率和法拉第效率(FE)的高效电催化剂对提高合成氨效率至关重要. 通过界面工程和空位工程来调节电子结构是提高电催化剂性能的一种新途径, 制备了具备核壳异质界面结构和硫空位的Cu2S@Co3S4/CF催化剂, 该催化剂表现出122 μg•h-1•cm-2的氨产率, 法拉第反应效率为34.6%, 并且经过6次循环测试后性能没有发生衰减, 优于大多数已报道的催化剂, 结合实验和表征可以发现, 该催化剂的高性能归因于Cu2S核可以通过均匀界面向表面Co3S4提供丰富的电子, 促进氮还原加氢反应, 并限制了*H耦合形成氢气, 抑制了竞争的析氢反应(HER), 有助于在氨产量和法拉第效率之间的更理想的平衡, 并且硫空位的引入进一步促进了N2的吸附和活化. 此外, Cu2S@Co3S4/CF作为阴极电催化剂应用于锌-氮气电池中表现出14.6 mW•cm-2的出色功率密度, 能够在电化学合成NH3的同时实现储能, 这项工作展示了一种很有前途的开发高效电催化剂的方法, 对解决能源危机和促进向低碳经济的转变具有重要意义.

郭超凡 , 苏进展 , 郭烈锦 . 具有内置电场效应的Cu2S@Co3S4/CF核壳异质结催化剂氮还原性能研究[J]. 化学学报, 2025 , 83(7) : 716 -724 . DOI: 10.6023/A25030091
In the context of the energy crisis and sustainable development, the electrocatalytic nitrogen reduction reaction (NRR) for ammonia synthesis with zero-carbon emission characteristics holds great promise. Developing highly efficient electrocatalysts with high ammonia yield and Faradaic efficiency (FE) for the nitrogen reduction reaction process (NRR) is crucial for enhancing the efficiency of ammonia synthesis. The modulation of electronic structure through interface engineering and vacancy engineering is a new approach to enhance the performance of catalyst. An efficient Cu2S@Co3S4/CF catalyst with core-shell heterojunction structure and sulfur vacancies was prepared, which exhibited an ammonia yield of 122 μg•h-1•cm-2 and a Faradaic efficiency of 34.6%, with the ammonia synthesis rate remaining consistently stable over 6 cycles of testing, outperforming most reported similar catalysts. Comprehensive experiments and characterization demonstrate that the high performance of the catalyst is attributed to the fact that the Cu2S core donates electrons to the Co3S4 shell through a uniform interface, which can promote the hydrogenation reactions of NRR while retarding the coupling of *H to form byproduct hydrogen. This contribute to a more optimal balance between the rate of ammonia production and the Faraday efficiency. Additionally, the formation of abundant sulfur vacancies significantly enhances nitrogen adsorption performance in this material. Moreover, the Zn‐N2 battery assembled with Cu2S@Co3S4/CF shows an excellent power density of 14.6 mW•cm−2, which enables the simultaneous ammonia production and energy supply. This work demonstrates a promising approach to develop highly efficient electrocatalysts for sustainable ammonia production, with significant implications for addressing the energy crisis and promoting the transition towards a low-carbon economy.

Key words: catalyst; nitrogen reduction reaction; core-shell structure; heterojunction; vacancy
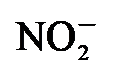 还原为NH3. Yang等[21]制备了CoP@Co核壳催化剂, 研究发现通过改变催化剂结构可以优化催化剂的合成氨反应性能. 上述研究表明, 钴基催化剂适用于NRR反应.
还原为NH3. Yang等[21]制备了CoP@Co核壳催化剂, 研究发现通过改变催化剂结构可以优化催化剂的合成氨反应性能. 上述研究表明, 钴基催化剂适用于NRR反应.| [1] |
(郭烈锦, 科技导报, 2021, 39, 1.)
|
| [2] |
(张谭, 余钟亮, 余嘉祺, 万慧凝, 包成宇, 涂文强, 杨颂, 化学学报, 2022, 80, 788.)
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
(詹溯, 章福祥, 化学学报, 2021, 79, 146.)
|
| [9] |
(张晓方, 甘汶, 纪之骄, 许明, 李初福, 何广利, 化工进展, 2025, 44, 809.)
|
| [10] |
(熊昆, 陈伽瑶, 杨娜, 蒋尚坤, 李莉, 魏子栋, 化学学报, 2021, 79, 1138.)
|
| [11] |
|
| [12] |
|
| [13] |
(姚嫣, 付年凯, 有机化学, 2025, 45, 1.)
|
| [14] |
(叶增辉, 刘华清, 张逢质, 有机化学, 2024, 44, 840.)
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(应霞薇, 浮建军, 曾敏, 刘文, 张天宇, 沈培康, 张信义, 化学学报, 2022, 80, 503.)
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
(王亚飞, 徐良晨, 姚向昱, 闫霆, 潘卫国, 当代化工研究, 2024, 15.)
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
/
| 〈 |
|
〉 |