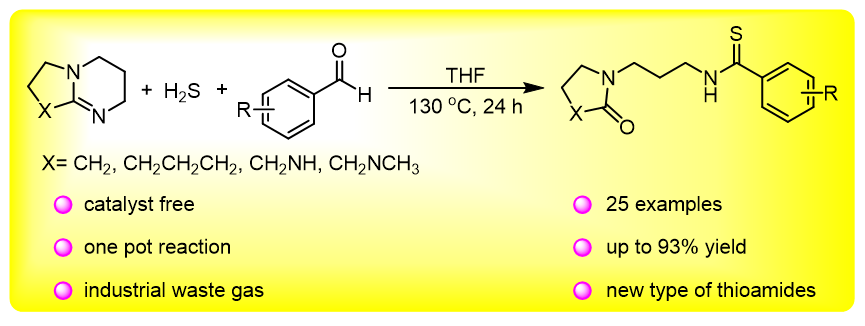
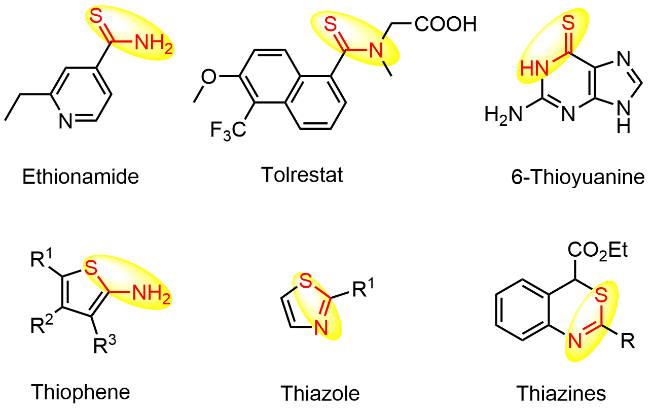
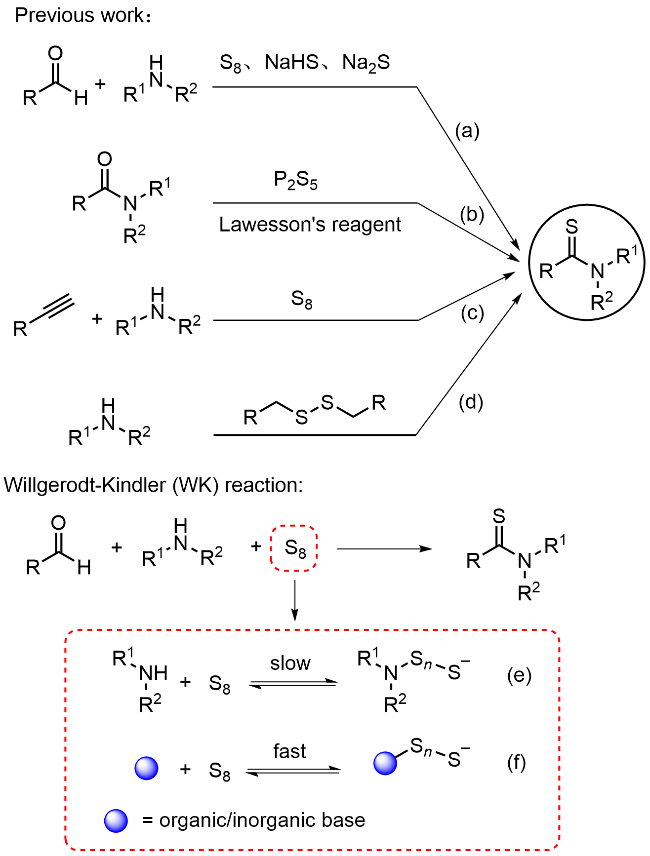
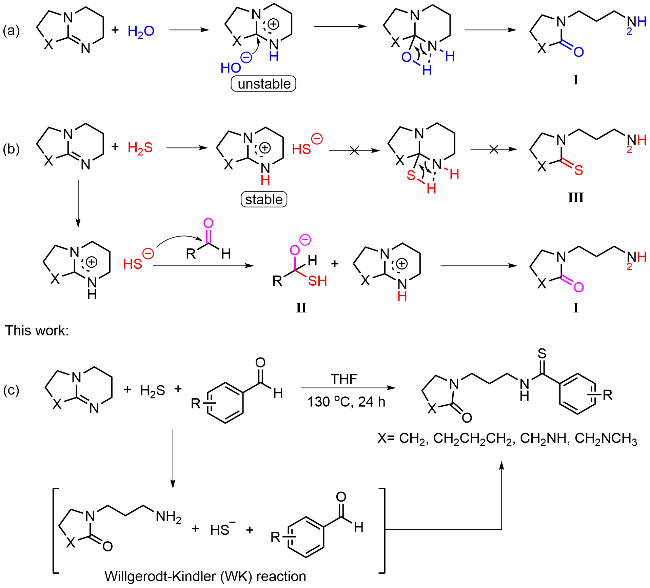
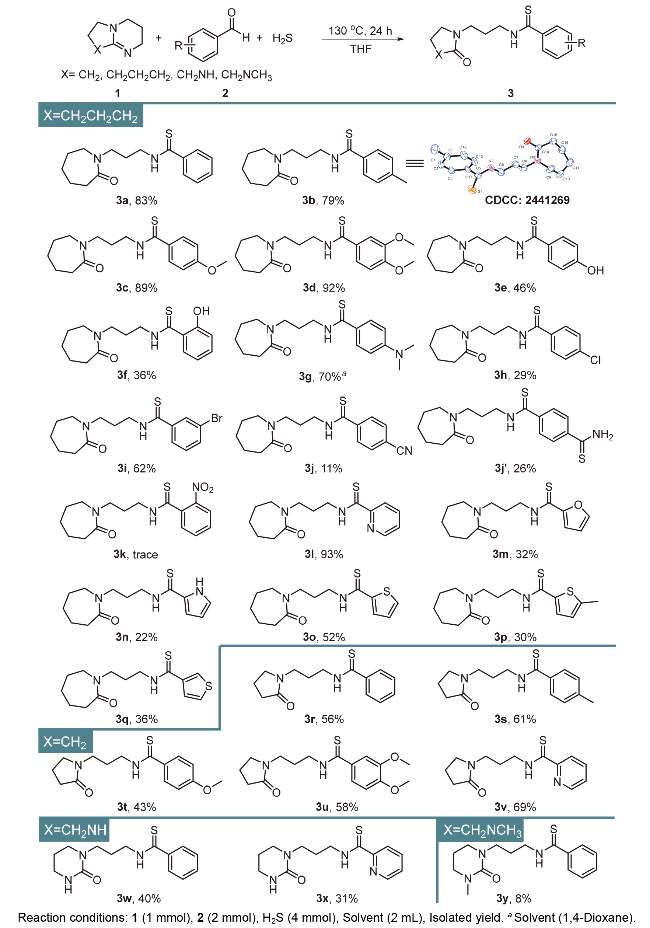
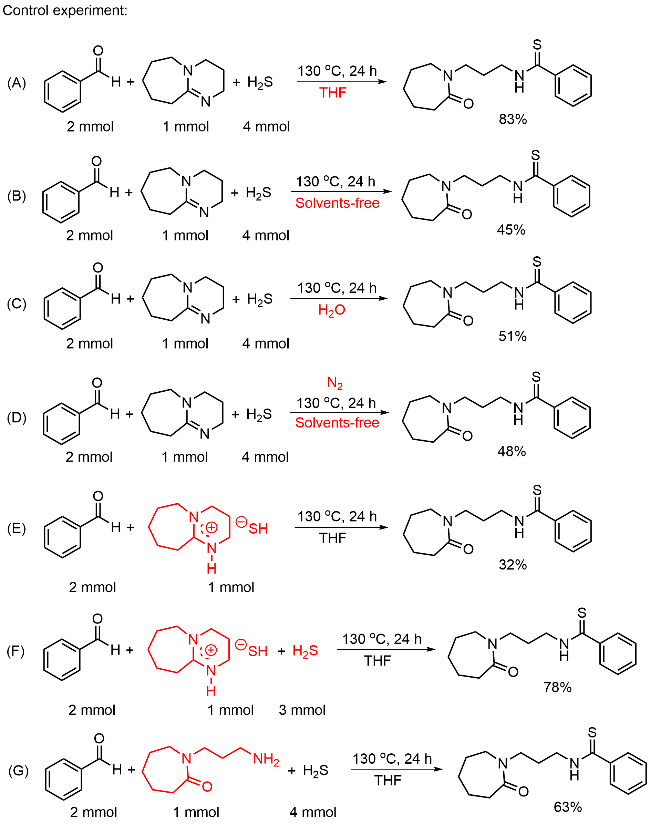
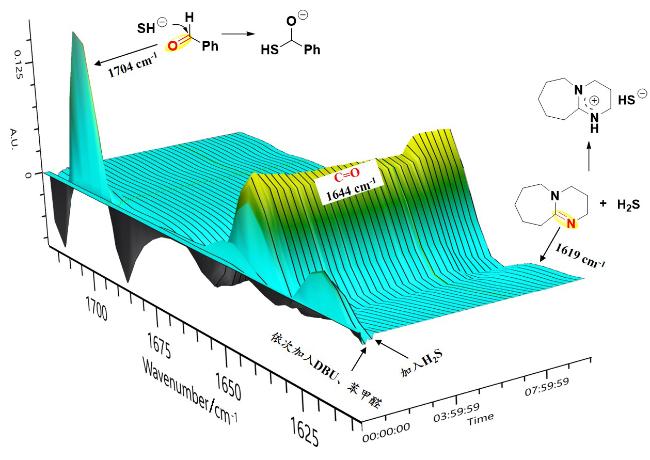
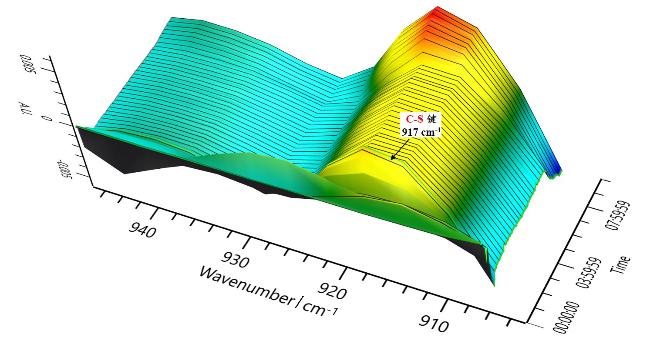
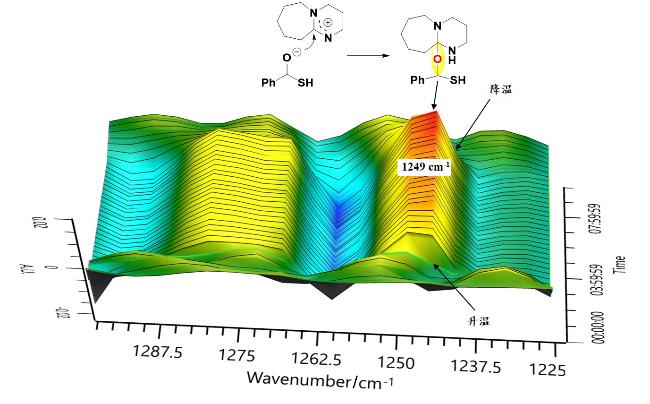
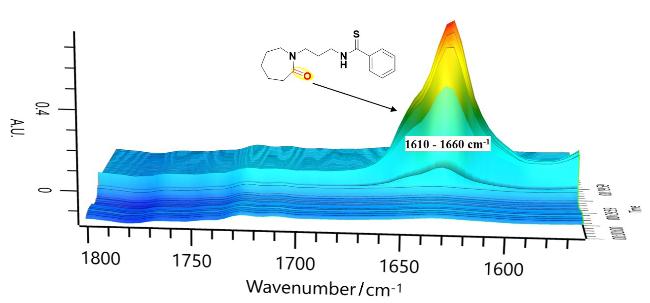
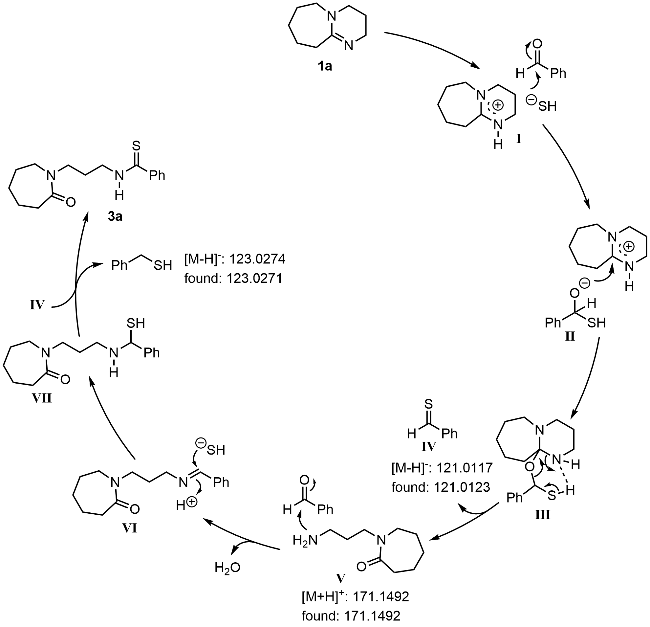
本研究以DBU (
1a)和苯甲醛(
2a)为模型底物, H
2S为硫源, 系统考察了投料比、温度、溶剂和时间对反应的影响规律(
Table 1). 首先对投料比进行了优化(
Table 1, Entries 1~8), 实验结果表明, 当DBU、苯甲醛和H
2S的投料物质的量比为1∶2∶4时, 反应产率最高达83% (
Table 1, Entry 4). 随后考察了温度对反应的影响规律(
Table 1, Entries 4, 9~14), 随着反应温度升高, 产率呈现先增加后减小的趋势. 当反应温度升高至130 ℃时, 反应产率高达83% (
Table 1, Entry 4). 由于该反应涉及气液两相体系, 溶剂的选择至关重要. 实验发现, 四氢呋喃(
Table 1, Entry 4)、1,4-二氧六环(
Table 1, Entry 15)等醚类溶剂表现出良好的反应效果. 相比之下, 聚乙二醇400 (
Table 1, Entry 16)作为溶剂时, 由于其高粘度不利于反应进行, 反应产率只有20%. 当使用
N,
N-二甲基甲酰胺(DMF)作为溶剂时(
Table 1, Entry 17), 产率降低至8%, 且在分离过程中检测到
N,
N-二甲基硫代苯甲酰胺, 推测原因可能是DMF在高温下分解生成二甲胺, 二甲胺会与苯甲醛、H
2S反应生成
N,
N-二甲基硫代苯甲酰胺, 使反应产率降低. 当溶剂为
N-甲基吡咯烷酮(NMP)时(
Table 1, Entry 18), 产率只有35%. 由于二甲基亚砜(DMSO)本身具有氧化性, 其作为溶剂可能将苯甲醛氧化为苯甲酸
[53], 导致没有产物生成(
Table 1, Entry 19). 当溶剂为乙腈时(
Table 1, Entry 20), 其腈基易受HS⁻亲核进攻, 不利于反应进行. 综合比较, 最终选定THF作为最佳反应溶剂. 最后, 考察了反应时间对产率的影响规律(
Table 1 Entry 4, 21~23). 结果表明, 随着反应时间延长, 产率呈现先增加后趋于平稳的趋势, 当反应时间达到24 h时, 产率稳定在83%.