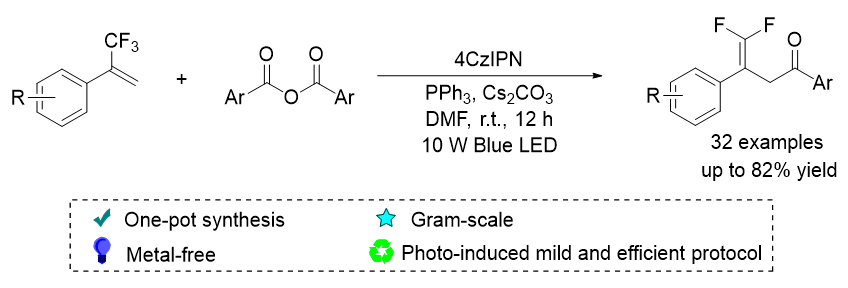
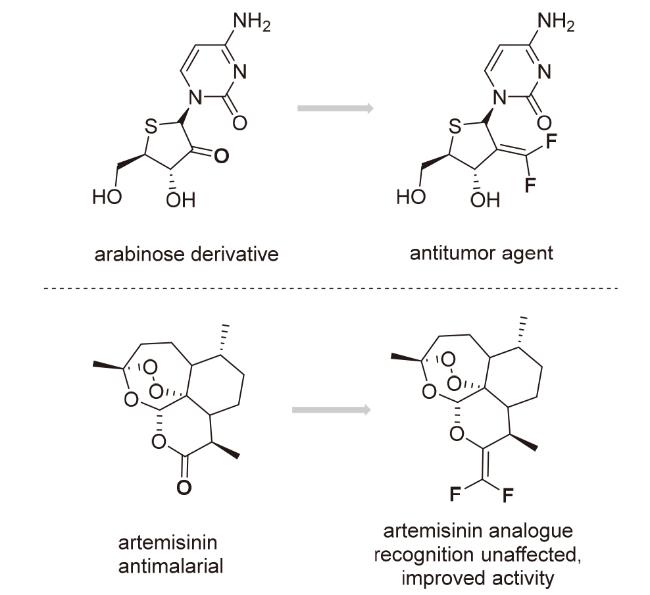
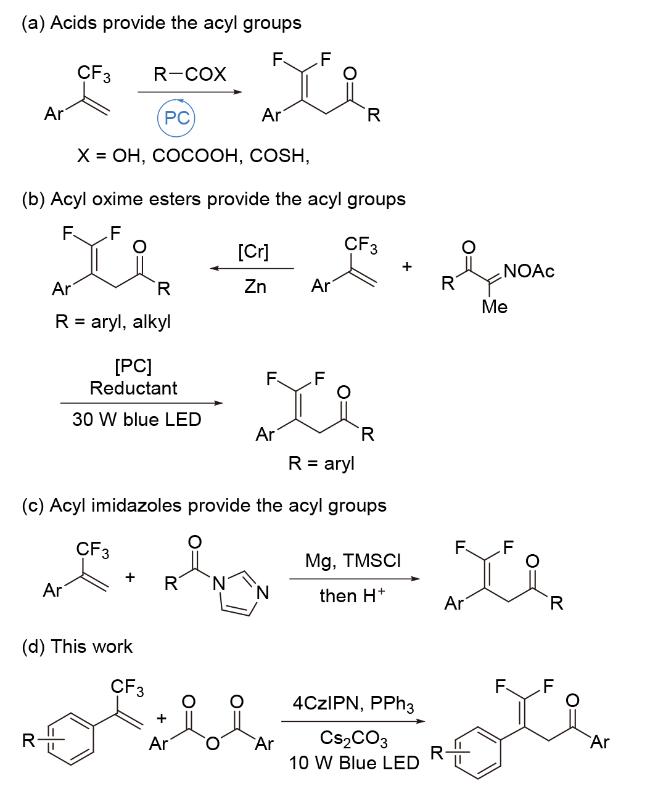
1 引言
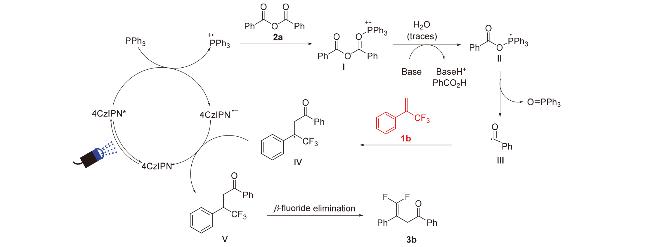
2 结果与讨论
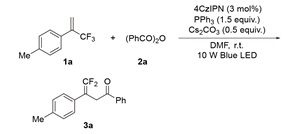
2.1 反应条件优化
表1 反应条件优化aTable 1 Conditions optimization |
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 82 |
| 2 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 or Eosin Y was used | 43, 16 |
| 3 | Ru(bpy)3Cl2 was used | N.R. |
| 4 | DBU or DIPEA was used | 46, 50 |
| 5 | NaHCO3 or K2CO3 was used | 36, 38 |
| 6 | EA, MeOH, DCE or DMSO was used | 34, Trace, 33, 30 |
| 7 | Addition 4 Å molecular sieves | 64 |
| 8 | No PC, or no Cs2CO3 | trace |
| 9 | No light, or no Ph3P | N.R. |
| 10 | Under air | N.R. |
| 11 | λ=390~395 nm | 69 |
a Unless otherwise noted, the standard reaction conditions were as follows: 1a (0.15 mmol), 2a (0.3 mmol), PC (0.0045 mmol), Ph3P (0.225 mmol), and base (0.075 mmol) in solvent (1.5 mL) with irradiation by 10 W Blue LEDs (λ=455~460 nm) under N2 at room temperature for 12 h. b Isolated yield. N.R.: no reaction. |
2.2 反应底物拓展
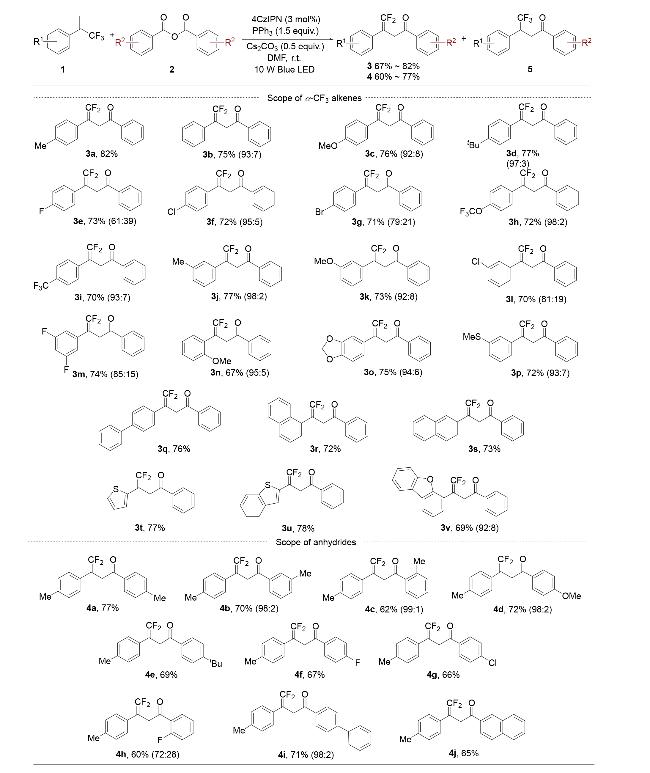
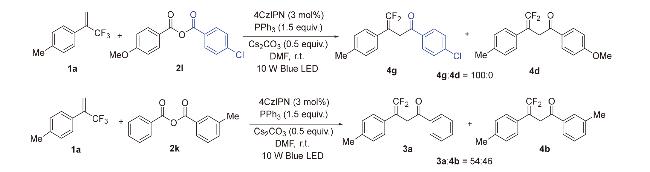
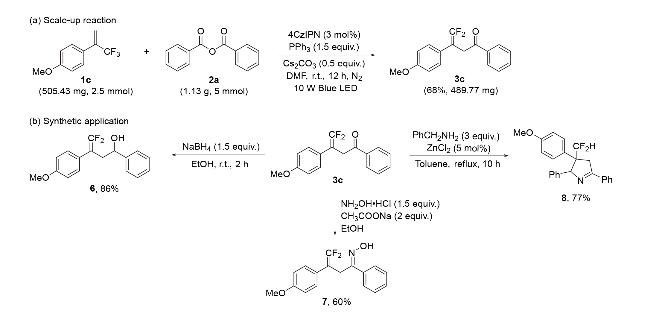
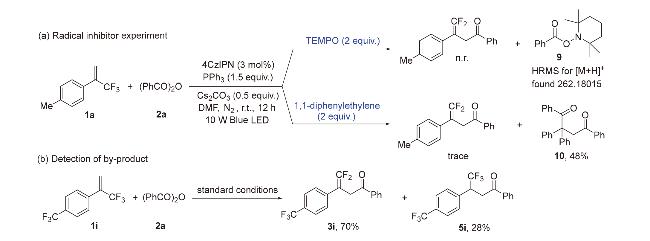
图3 底物范围Figure 3 Substrate scope a Reaction conditions: 1a~1v (0.3 mmol), 2a~2j (0.6 mmol), 4CzIPN (0.009 mmol), PPh3 (0.45 mmol), and Cs2CO3 (0.15 mmol) in DMF (3 mL) under 10 W blue LED light, under nitrogen atmosphere at room temperature for 12 h; the ratio of product 3 or 4 to byproduct 5 is given in parentheses, determined by 19F NMR. b Isolated yield. |