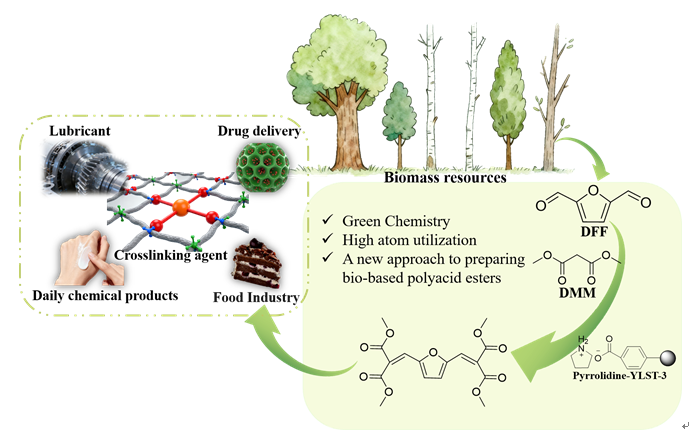
Biomass resources, characterized by their wide distribution, carbon neutrality, and renewability, have emerged as ideal alternatives to fossil fuels. Derived from these resources, biobased platform compounds such as 5-hydroxymethylfurfural (HMF) and 2,5-diformylfuran (DFF) exhibit diverse application potential. These biobased platform compounds can be converted into high-value-added chemicals through organic chemical reactions. Bio-based polyacid esters show broad potential for applications in fields such as chemical engineering, polymer materials, and biomedicine, where they can serve as crosslinking agents, lubricants, emulsifiers, among other roles. Conventional methods for synthesizing polybasic acid esters typically involve strong oxidants, intricate processes, and demanding equipment requirements. In this study, we prepared a series of supported ionic liquid catalysts via in-situ acid-base neutralization reaction, employing weak acidic ion exchange resin as the solid support and organic amines as functional modifiers, and used them to the preparation of bio-based polyacid esters. When 2,5-diformylfuran (DFF) and Dimethyl malonate (DMM) were employed as substrates, the yield of bio-based polyacid esters can reach 99.2% in the presence of Pyrrolidine-YLST-3. The structural modifications of the resins were comprehensively characterized using multiple analytical techniques, including Elemental Analysis (EA), Nuclear Magnetic Resonance(NMR), Fourier Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS). These comprehensive analyses provided conclusive evidence for the successful surface modification of YLST-3 resin with pyrrolidine, revealing distinct chemical interactions between the modifier and the resin. Comprehensive characterization of both fresh and deactivated catalysts was performed using EA, FTIR, and XRD to investigate catalyst deactivation mechanisms. Successful regeneration was accomplished through a two-step protocol involving hydrogen peroxide oxidation followed by pyrrolidine re-modification, which substantially improved cycling stability. Substrate scope evaluation demonstrated that the Pyrrolidine-YLST-3 catalyst exhibited excellent activity in Knoevenagel condensation reactions across diverse substrates, confirming its broad applicability.
[1] Kaygusuz K.Energy Sources, Part B. 2007, 2, 73.
[2] Qiao X. L.; Murshed M.; Alam M. M.; Das N.; Khudoykulov K.; Tariq S.Gondwana Res. 2024, 126, 355.
[3] Deng Y.; Xing C. Y.; Xie X. D.; Cai L.Sustain. Comput.: Inform. Syst. 2022, 36, 100782.
[4] Ayed C.; Huang W.; Kizilsavas G.; Landfester K.; Zhang K. A.I.ChemPhotoChem. 2020, 4, 571.
[5] Eseyin A. E.; Steele P. H.Int. J. Adv. Chem. 2015, 3, 42.
[6] Edumujeze D.;Fournier-Salaün, M.-C.; Leveneur, S.Fuel. 2025, 381, 133423.
[7] Ju J. H.; Xu J. L.; Wang K. J.; Huang J. H.Acta Chim. Sin. 2024, 82, 1216(in Chinese).
(鞠嘉浩,徐吉磊,王康军,黄家辉,化学学报,2024,82,1216.)
[8] Sun W. Q.; Xu J. L.; Li L.; Liu Z.; Xiao Z. Y.; An Q. D.; Huang J. H.Mol. Catal. 2025, 573, 114842.
[9] Han Y. N.; Xu J. L.; Li B. Z.; An Q. D.; Xiao Z. Y.; Huang J. H.ChemCatChem. 2025, 17, e02127.
[10] Kwiatkowska M.; Kowalczyk I.; Szymczyk A.Mater. Today Commun. 2019, 20, 100577.
[11] Bouyahya C.; Patrício R.; Paço A.; Lima M. S.; Fonseca A. C.; Rocha-Santos, T.; Majdoub, M.; Silvestre, A. J. D.; Sousa, A. F.Polymers. 2022, 14, 3868.
[12] Xin C.; Jiang J.; Deng Z. W.; Ou L. J.; He W. M.Acta Chim. Sin. 2024, 82, 1109(in Chinese).
(辛翠,蒋俊,邓紫微,欧丽娟,何卫民,化学学报, 2024, 82, 1109.)
[13] Guo J. R.; Zhang S. Y.; He J. H.; Ren S. X.Acta Chim. Sinica. 2024, 82, 242(in Chinese).
(郭建荣,张书玉,贺军辉,任世学,化学学报,2024, 82, 242.)
[14] Iroegbu A. O.; Sadiku E. R.; Ray S. S.; Hamam Y.Chem. Afr. 2020, 3, 481.
[15] Demirbas M. F.; Balat M.; Balat H.Energy Convers. Manage. 2009, 50, 1746.
[16] Obermeier F.; Hense D.; Stockmann P. N.; Strube O. I.Green Chem. 2024, 26, 4387.
[17] Damle S.; Madankar C.Tenside Surfact. Deterg. 2023, 60, 611.
[18] Tang L. S.; He X. Y.; Huang R.Int. J. Mol. Sci. 2025, 26, 727.
[19] Zhu X. Y.; Jiang P. P.; Leng Y.; Lu M. J.; Lv Z. H.; Li Y. C.; Li Z. H.J. Vinyl Addit. Technol. 2024, 30, 1066.
[20] Fiume M. M.; Bergfeld W. F.; Belsito D. V.; Hill R. A.; Klaassen C. D.; Liebler D. C.; Marks J. G.; Shank R. C.; Slaga T. J.; Snyder P. W.; Gill L. J.; Heldreth B.Int. J. Toxicol. 2023, 42, 5S.
[21] Illa O.; Serra A.; Ardiaca A.; Herrero X.; Closa G.; Ortuño R. M.Int. J. Mol. Sci. 2019, 20, 4333.
[22] Perez F. M.; Gatti M. N.; Fermanelli C. S.; Saux C.; Renzini M. S.; Pompeo F.Next Mater. 2024, 2, 100125.
[23] Yanagawa F.; Sugiura S.; Takagi T.; Sumaru K.; Camci‐Unal, G.; Patel, A.; Khademhosseini, A.; Kanamori, T.Adv. Healthcare Mater. 2014, 4, 246.
[24] Nagendramma P.Lubr. Sci. 2011, 23, 355.
[25] Wei J. J.; Zhu L. L.; Lu Q. Y.; Li G. C.; Zhou Y. L.; Yang Y. M.; Zhang L. Z.J. Controlled Release. 2023, 354, 337.
[26] Picataggio S.; Rohrer T.; Deanda K.; Lanning D.; Reynolds R.; Mielenz J.; Eirich L. D.Bio-Technol. 1992, 10, 894.
[27] Han L.; Peng Y. F.; Zhang Y. Y.; Chen W. J.; Lin Y. P.; Wang Q. H.Front. Microbiol. 2017, 8, 2184.
[28] Kulsrestha G. N.; Shankar U.; Sharma J. S.; Singh J.J. Chem. Technol. Biotechnol. 2007, 50, 57.
[29] Tian H.; Zhang Y. J.; Yu D.; Yang X.; Wang H.; Matindi C.; Yin Z.; Hui H. S.; Mamba B. B.; Li J. X.Electrochim. Acta. 2022, 426, 140796.
[30] Matsumura Y.; Yamamoto Y.; Moriyama N.; Furukubo S.; Iwasaki F.; Onomura O.Tetrahedron Lett. 2004, 45, 8221.
[31] Gauthard F.; Horvath B.; Gallezot P.; Besson M.Appl. Catal., A. 2005, 279, 187.
[32] Lisicki D.; Orlinska B.Pol. J. Chem. Technol. 2018, 20, 102.
[33] Ribeiro A. P.C.; Spada, E.; Bertani, R.; Martins, L. M. D. R. S.Catalysts. 2020, 10, 1443.
[34] Li J.; Liu Y.; Gan F. F.; Yang Y. X.Chin. J. Anal. Lab. 2017, 36, 726.
[35] Hu Y. C.; Yuan L.; Zhang X. L.; Zhou H.; Wang P.; Li G.; Wang A. Q.; Cong Y.; Zhang T.; Liang X. M.; Li W.; Li N.ACS Sustainable Chem. Eng. 2019, 7, 2980.
[36] Yuan L.; Hu Y. C.; Li G. Y.; Han F. G.; Wang A. Q.; Cong Y.; Zhang T.; Wang F.; Li N.Green Energy Environ. 2024, 9, 1267.
[37] Liu S. S.; Dong W. W.; Li Z. Z.; Zhang Y. Y.; Li C.; Jiao L. Y.Acta Chim. Sinica. 2025, 83, 479(in Chinese).
(刘珊珊, 董微微,李珍珍,张瑶瑶,李超,焦林郁,化学学报,2025,83, 479.)
[38] Sood K.; Saini Y.; Thakur K. K.Mater. Today: Proc. 2023, 81, 739.
[39] Aggarwal A.; Singh A.; Chopra H. K.Curr. Org. Chem. 2023, 27, 130.
[40] Ma M.; Li H. S.; Yang W.; Wu Q.; Shi D. X.; Zhao Y.; Feng C. H.; Jiao Q. Z.Catal. Lett. 2017, 148, 134.
[41] Yao N.; Tan J.; Liu X.; Liu Y.; Hu Y. L.J. Saudi Chem. Soc. 2019, 23, 740.
[42] Rui Li, J.; Chen, C.; Hu, Y. L.ChemistrySelect. 2020, 5, 14578.
[43] Korhonen H.; Helminen A. O.; Seppälä J. V.Macromol. Chem. Phys. 2004, 205, 937.
[44] Wójcika M. J.; Tatara W.; Boczar M.; Apola A.; Ikeda S.J. Mol. Struct. 2001, 596, 207.
[45] Galkina Y. A.; Kryuchkova N. A.; Vershinin M. A.; Kolesov B. A.J. Struct. Chem. 2017, 58, 911.
[46] Herbert J. M.;Head-Gordon, M.J. Am. Chem. Soc. 2006, 128, 13932.
[47] Lees R. M.; Sun Z. D.; Billinghurst B. E.J. Chem. Phys. 2011, 135, 104306
[48] Hasnat A.; Juvekar V. A.AIChE J. 2004, 42, 161.
[49] Kwan Y. C.G.; Ng, G. M.; Huan, C. H. A.Thin Solid Films. 2015, 590, 40.
[50] Rushdi A. I.; Simoneit B. R.T.Astrobiology. 2004, 4, 211.
[51] Su M. D.; Liu Y. F.; Nie Z. W.; Yang T. L.; Cao Z. Z.; Li H.; Luo W. P.; Liu Q.; Guo C. C.J. Org. Chem. 2022, 87, 7022.
[52] Masoudi-Khoram, M.; Zargarian, M.; Nematollahi, D.; Zolfigol, M. A.; Sepehrmansourie, H.; Khazalpour, S.Electrochim. Acta. 2023, 437, 141512.
[53] Wang L.; Xu G.; Xiao J.; Tao M. L.; Zhang W. Q.Ind. Eng. Chem. Res. 2019, 58, 12401.
[54] Zhao S. X.J. Mol. Struct. 2018, 1167, 11.
[55] Anbu N.; Maheswari R.; Elamathi V.; Varalakshmi P.; Dhakshinamoorthy A.Catal. Commun. 2020, 138, 105954.
[56] Sobrinho R. C.M. A.; Oliveira, P. M. d.; D'Oca, C. R. M.; Russowsky, D.; D'Oca, M. G. M.RSC Adv. 2017, 7, 3214.
[57] Hu X. M.; Ngwa C.; Zheng Q. G.Curr. Org. Synth. 2016, 13, 101.



